Introduction
Over the past 25 – 30 years, researchers in the bio-medical community have built up an extensive knowledge base about the absorption, safety, and health effects of Coenzyme Q10. Coenzyme Q10 is a substance that is both synthesized in the body and absorbed from food sources; however, the body’s synthesis of Coenzyme Q10 peaks in a person’s 20’s and then declines. Moreover, the use of statin medications inhibits the body’s production of Coenzyme Q10. It is very difficult if not impossible to compensate in the diet alone for the Coenzyme Q10 deficits caused by increasing age and statin medications.
The cumulative research results show that Coenzyme Q10, when taken as a nutritional supplement or as a medicine, is safe and well-tolerated. It is necessary for the cellular production of ATP (adenosine triphosphate) molecules, the basic source of energy in the body. It is necessary as an antioxidant to neutralize harmful free radicals. It is necessary to protect the smooth functioning of the endothelium, the inner lining of the blood vessels and lymph vessels.
Research results now show that adjuvant treatment with Coenzyme Q10 improves symptoms and survival in patients with chronic heart failure [64,70], reduces the number and severity of complications following heart surgery [39,55], and helps to lower high blood pressure [2,78].
In this history of Coenzyme Q10 research, the following abbreviations are used:
- kg … kilogram
- mg … milligram
- mcg … microgram
- mL … milliliter
- L … liter
Coenzyme Q10: A Substance with Many Names
There are many histories that can be written about the discovery and development of the redox chemical substance that is known by many names:
- Coenzyme Q10
- Ubiquinone = oxidized Coenzyme Q10
- Ubiquinol = reduced Coenzyme Q10
- Q or Q10 or CoQ10
- Ubidecarenone
- Myoqinon
- Bio-Quinone Q10
- 2,3-Dimethoxy-5-methyl-6-decaprenyl-1,4-benzoquinone
Illustration of the Coenzyme Q10 molecule – Coenzyme Q10 is found in the mitochondria of all healthy cells in the body except in the red blood cells – Coenzyme Q10 affects everyone positively, and it has no toxic effects at all
There are many important people, scientists and biomedical researchers and medical doctors, who can and should be emphasized and celebrated in any history of Coenzyme Q10.
Coenzyme Q10 Researchers
In this particular history of Coenzyme Q10 research, we choose to tell Coenzyme Q10’s story with an emphasis on two very important men in the story world-wide and, then, with lesser emphasis on the two men behind the founding and managing of the Danish company Pharma Nord, the producer of the Bio-Quinone Q10 and Myoqinon preparations.
The lives and careers of the two sets of men intersected for many years and were interwoven around a mutual commitment to the development of a well-absorbed nutritional supplement and adjuvant medical therapy drug from the substance Coenzyme Q10.
Dr. Karl August Folkers, 1906 - 1997
The first of the two great men in the history of Coenzyme Q10 research was the American research chemist and visionary Dr. Karl August Folkers. Dr. Folkers was a researcher who seemed able to visualize in his mind the pathways of biochemical reactions whenever he looked at the chemical formulas and structures of naturally occurring substances. He foresaw more clearly than anyone the potential benefits of Coenzyme Q10 supplementation in patients suffering from heart disease and cancer.
Using his knowledge of chemistry and biology, Dr. Folkers could see the potential health effects of Coenzyme Q10. He could imagine great break-throughs if only the funding for biomedical research could be secured. In the words of Sven Moesgaard, Dr. Folkers was the chemist who dared dream Coenzyme Q10 castles in the air.

Sven Moesgaard of Pharma Nord and Dr. Karl Folkers (right) – In front of the Tycho Brahe Planetarium, Copenhagen 1992
Visualizing Coenzyme Q10 at the cellular level
What Dr. Folkers visualized better than anyone else was the biochemical activity of the substance Coenzyme Q10 in the mitochondria of human cells. Dr. Folkers knew that the mitochondria are the site in the cells in which the synthesis of adenosine triphosphate (ATP) takes place. ATP is the carrier of chemical energy in the cells. At the same time, Dr. Folkers knew that the mitochondria are a major producer of harmful free radicals in the body and need antioxidant protection.
Dr. Folkers could see that Coenzyme Q10, a redox substance known as ubiquinone in its oxidized form and as ubiquinol in its reduced form, was an essential bio-nutrient. The ubiquinone form is a component of the electron transport chain in the process of aerobic cellular respiration that produces and transfers chemical energy in the form of ATP. The ubiquinol form is a powerful antioxidant that can quench reactive oxygen species.
Dr. Svend Aage Mortensen, 1942 - 2015
The second of the two great men in the history of Coenzyme Q10 research was the Danish cardiologist and researcher Dr. Svend Aage Mortensen. Dr. Mortensen developed the rationale for and designed and led the multi-center Q-Symbio study that was completed and published in 2014.
Q-Symbio met the gold standard for a test of Coenzyme Q10 as an adjunctive treatment of chronic heart failure [70]. It was a randomized, double-blind, placebo-controlled clinical trial with emphasis on the heart failure patients’ disease symptoms, bio-markers, and long-term outcomes (hospitalization rates and mortality rates, in particular).
Heart failure is often referred to as congestive heart failure or chronic heart failure. The condition is increasingly prevalent, and it has a generally poor prognosis. It is the medical condition that occurs whenever the heart muscle, in its contractions, is not able to pump out a sufficient volume of blood to meet the body’s needs for oxygen and nutrients. Typical symptoms include shortness of breath, early onset of fatigue, swelling in the legs, and possibly chest pain. Common causes of heart failure are coronary artery disease, high blood pressure, atrial fibrillation, leaky heart valves, and infection and inflammation as well as unknown causes.
From Dr. Mortensen’s perspective, a very plausible cause of heart failure is the energy starvation of the heart muscle cells. Whenever the concentration of Coenzyme Q10 in the heart muscle cells is abnormally low, the heart muscle will be starved for energy. Dr. Mortensen suspected dysfunction of the bioenergetics process in the heart muscle cells, a condition in which the mitochondria of the heart muscle cells lack an adequate supply of Coenzyme Q10 and fail to synthesize enough ATP. Dr. Mortensen was determined to provide clinical research evidence for the plausibility of this explanation of the cause of heart failure.
Dr. Mortensen was, in the words of his fellow Coenzyme Q10 researcher, Sven Moesgaard, the man who built a good solid foundation under the Coenzyme Q10 castles in the air that Dr. Folkers had dreamed. More than any one other researcher, Dr. Mortensen provided the rationale and the empirical evidence for adding Coenzyme Q10 supplementation as an adjuvant treatment to the guidelines for the standard treatment of chronic heart failure.

Dr. Karl Folkers and Dr. Svend Aage Mortensen (right)
The conventional treatment of chronic heart failure is designed mostly to block neurohormonal responses in patients. In the words of Dr. Mortensen, conventional heart failure therapies block rather than enhance cellular processes. What adjuvant treatment with Coenzyme Q10 adds to the treatment regimen is support and enhancement of the cellular bioenergetics of the failing heart.
Who was Dr. Karl Folkers?
Dr. Folkers was such a capable chemist and biochemist that, when Dr. Folkers died, Professor William Shive, himself a prominent chemist, wrote the biographical memoir of Dr. Folkers that was published by the National Academy Press [81]. Dr. Shive emphasized not only Dr. Folkers’ contributions to research science but also his assistance to and collaboration with many other researchers.
It was a big part of Dr. Folkers’ dream to see Coenzyme Q10 become a component of medical education and of clinical practice. His major research interests were first, last, and always the biochemical study of disease conditions and the use of nutritional supplements to promote health and well-being. Initially, Dr. Folkers seems to have thought that the substance Coenzyme Q10 was a vitamin, Vitamin Q, as it were.
Coenzyme Q10 vitamin-like and essential but not a vitamin
In imagining that Coenzyme Q10 would prove to be a vitamin, Dr. Folkers thought, at first, that Coenzyme Q10 was another one of those organic compounds that are essential for human growth and development but which the body is unable to synthesize. Very soon, he realized that yes, the human body does synthesize Coenzyme Q10. However, further research revealed that the body’s production of the substance peaks in a person’s 20s and then decreases with increasing age (Kalen).
The bio-synthesis of Coenzyme Q10 continues to decrease to the extent that a 65-year-old man’s synthesis of Coenzyme Q10 is likely to be half or less than a 25-year-old man’s synthesis of the substance is. Moreover, Dr. Folkers realized and could demonstrate early on that the statin medications used to inhibit the bio-synthesis of cholesterol were also inhibiting the bio-synthesis of Coenzyme Q10. He could envision the conditions for and the consequences of Coenzyme Q10 deficiency.
By 1990, he was able to publish the results of three studies that demonstrated that the statin medication lovastatin, while successful at lowering patients’ cholesterol levels, was also inducing Coenzyme Q10 deficiencies in patients.
Dr. Folkers in the early years at Merck: B vitamins and anti-biotics
Dr. Folkers joined the American pharmaceutical company Merck (later Merck, Sharp and Dohme) as a research chemist in 1934 and did research there, with ever increasing responsibility, until 1963. He and his research team first did ground-breaking work on the structure and synthesis of vitamin B6 followed by similar research on other B vitamins, pantothenic acid and biotin.
Dr. Folkers next got deeply involved in the isolation and structure determination of the anti-biotic streptomycin. Then, in the work for which he became especially well-acclaimed, he and his research team determined the chemical structure of vitamin B12 and made its commercial preparation possible.
The mevalonate pathway
While still at Merck, Dr. Folkers and his research team succeeded in the discovery, isolation, and synthesis of mevalonic acid, which is the biochemical precursor of the substances produced in the body in the mevalonate pathway, namely cholesterol, ubiquinone, and dolichol. The work of the Merck team led directly to the development of approaches to limiting the biosynthesis of cholesterol. Later, it was Dr. Folkers’ understanding of the production of cholesterol and Coenzyme Q10 in the same biological pathway that would make him realize that cholesterol inhibiting drugs also inhibit Coenzyme Q10 production.
A simplified illustration of the mevalonate pathway producing the building blocks used to make isoprenoids. It is also seen why statins inhibit the body's production of CoQ10
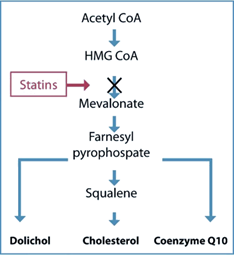
A simplified illustration of the mevalonate pathway producing the building blocks used to make isoprenoids. It is also seen why statins inhibit the body's production of CoQ10
Dr. Folkers and the work on Coenzyme Q10 at Merck
While Dr. Folkers was at Merck, making his reputation in the structural determination of natural substances, British and American researchers, working independently of one another, isolated a yellow fat-soluble quinone substance from animal tissue. In 1955, Dr. Festenstein and his colleagues, working in the Liverpool laboratory of Dr. R. A. Morton, isolated the substance. Because it seemed to be ubiquitous in animal tissue, Professor Morton called it ubiquinone.
In 1957, unaware of the British discovery and seeking to understand the role of the electron transport system in the production of ATP, Dr. Fred Crane and colleagues, working in the lab of Dr. David Green at the University of Wisconsin, isolated the yellow fat-soluble substance that they found in the mitochondria of extracts of beef heart tissue. They called the substance Coenzyme Q based on its role as a coenzyme in the electron transfer chain.
Dr. Crane takes samples of Coenzyme Q10 to Dr. Folkers
Dr. Crane, knowing of Dr. Folkers’ skills in the laboratory, took samples of the recently isolated substance to Merck in New Jersey where Dr. Folkers and his team were able, in 1958, to determine the structure of Coenzyme Q10. The Q in the name stands for the cyclic organic arrangement of the quinone head, and the 10 in the name stands for the number of isoprenoid units in the tail of the substance.

Dr. Fred Crane at the London CoQ10 Symposium 2002
Dr. Folkers and the role of Coenzyme Q10 in heart failure
In 1963, Dr. Folkers resigned from Merck and accepted a position as president and chief executive officer at the Stanford Research Institute. At Stanford, in the time that he could spare from his executive duties, Dr. Folkers continued to do some Coenzyme Q10 research. Then, in 1968, he moved to Austin, Texas, to take the position of director of the Institute of Biomedical Research where he would have more time and resources for research into the clinical effects of Coenzyme Q10.
In Austin, Dr. Folkers could finally concentrate his research focus on the medical applications of Coenzyme Q10 supplementation. Dr. Folkers and his fellow researchers became more and more convinced of the efficacy of supplementation with Coenzyme Q10 in a variety of diseases:
- Cardiomyopathy (disease of the heart muscle)
- Hypertension
- Muscular dystrophy
- Periodontal disease
1970s: Coenzyme Q10 for the treatment of heart failure in Japan
At some time in the early 1970s, Dr. Folkers took samples of Coenzyme Q10 to a biochemical meeting in Japan. There, he met a cardiologist, Dr. Yuichi Yamamura, whom he convinced to supplement heart failure patients with Coenzyme Q10. Dr. Yamamura became the first cardiologist to treat heart failure patients with Coenzyme Q10.
Initially, Dr. Folkers delivered Coenzyme Q10 that was extracted from fish livers in a small extraction plant that he had in Galveston, Texas. The Coenzyme Q10 that was extracted from fish livers obtained from the fishing industry in Galveston was purified and used in research studies in Japan and in the USA. For a time in the 1970s, Dr. Folkers was supplying all of the Coenzyme Q10 used for research in the USA. Dr. Folkers was, at that time, also investigating methods to extract Coenzyme Q10 from tobacco plants and methods to produce Coenzyme Q10 by a bacterial process (Pseudomonus arius). [information provided by Dr. William Judy]
Already in 1974, in recognition of the link between lower blood and tissue levels of Coenzyme Q10 and congestive heart failure, Japan approved the use of Coenzyme Q10 for the medical treatment of heart failure. Coenzyme Q10 was known to be safe and without any significant adverse effects. In 1977, then, the Kaneka Corporation in Japan began producing and distributing yeast-fermented Coenzyme Q10.

Orange Coenzyme Q10 crystals – A proper Coenzyme Q10 preparation needs to have the raw material dissolved in oils in a way that inhibits the re-crystallization of the Q10 molecules at body temperature.
1970s: Deficiency of Coenzyme Q10 in human heart disease
By the 1970s, Dr. Folkers and his team of researchers, which included the Italian researcher Gian Paolo Littarru, had solid evidence that patients with heart disease and patients undergoing heart surgery had blood and tissue Coenzyme Q10 levels significantly below normal levels [21]. The evidence came from tissue biopsy samples from more than 100 cardiac surgery patients and from blood samples from more than 1000 cardiac patients.

Left to right, Dr. Fred Crane, Dr. Karl Folkers, Dr. Gian Paolo Littarru at the Ancona CoQ10 Symposium 1996
1978: Dr. Peter Mitchell and the Nobel Prize for Chemistry
For his work done throughout the 1970s, the British chemist Dr. Peter Mitchell was awarded the Nobel Prize for Chemistry in 1978. The Royal Swedish Academy of Sciences honored Dr. Mitchell for his explanation of the role of Coenzyme Q10 in the biological transfer of energy in the cells through the movement of electrons to the electron transport chain in the inner mitochondrial membranes and through the movement of hydrogen ions (protons) across the inner membranes of the mitochondria.
Dr. Mitchell’s theoretical work on chemiosmosis as an explanation of the generation of ATP provided valuable underpinnings for the clinical work of Dr. Folkers and the cardiologists with whom Dr. Folkers collaborated. It also made the significance of the clinical research more apparent.

His Majesty King Carl Gustaf of Sweden presents the 1978 Nobel Prize in Chemistry to Dr. Peter Mitchell (left).
Who was Dr. Svend Aage Mortensen?
Dr. Svend Aage Mortensen – known as SAM to his colleagues and friends – was a very capable and yet very modest man whose passionate interest was the care and welfare of heart disease patients. His education and training in cardiology was wide, indeed, but chronic heart failure and chronic diseases of the heart muscle (cardiomyopathy) were his primary focus. His colleagues, in their memorial to him in the Danish medical journal Ugeskrift for Læger say that he was the best colleague and mentor that one could hope for.

Dr. Svend Aage Mortensen, Danish cardiologist and Coenzyme Q10 researcher, leader of the Q-Symbio study of Coenzyme Q10 adjuvant treatment and chronic heart failure
Dr. Mortensen finished his medical education in 1974. His first posting as a cardiologist was to Helsingør in northern Sjælland where he introduced the practice of acute pacemaker surgery. Then, in 1978, he was assigned to Rigshospitalet (later named Copenhagen University Hospital) in Copenhagen, and he remained in The Heart Center in Copenhagen until his unexpected death from complications following heart valve surgery in 2015.
During a study tour to London, Dr. Mortensen learned to conduct heart tissue biopsies. His use of the technique and its application provided the data for his subsequent Doctor of Science dissertation, which he defended in 1989.
On a study tour to Stanford University, Dr. Mortensen learned to do heart transplantation. In 1990, he was involved in the establishment of a heart transplantation program in Copenhagen, and, from the start, he was the medical officer in charge of the program.
From the early 1980s to 2015, Dr. Mortensen’s research efforts were concentrated on understanding the role of Coenzyme Q10 in the prevention and treatment of chronic heart disease. Fortunately, before his untimely death, Dr. Mortensen was able to complete and publish the results of the Q-Symbio study of the effect of Coenzyme Q10 supplementation on the morbidity and mortality of chronic heart failure patients. More about the Q-Symbio study later in this history of Coenzyme Q10.
1980s: Dr. Folkers collaborates with heart failure researchers
In the early 1980s, Dr. Folkers began to work with two cardiologists and a post-doctoral research fellow to test the effects of Coenzyme Q10 supplementation of heart disease patients. These three researchers were pioneers in the adjuvant treatment of heart disease with Coenzyme Q10:
- Dr. Svend Aage Mortensen of Copenhagen, Denmark
- Dr. Per Langsjoen of Temple, Texas
- Dr. William Judy of Indianapolis, Indiana
1980s: Folkers and Mortensen: Low heart muscle tissue levels of Coenzyme Q10
In 1984 and 1985, Dr. Mortensen and Dr. Folkers were able to demonstrate that the heart muscle tissue levels of Coenzyme Q10 in patients in the New York Heart Association classes III and IV were lower than the levels of heart muscle Coenzyme Q10 in patients in the NYHA classes I and II. With their tissue data, Dr. Folkers and Dr. Mortensen were beginning to establish a biochemical rationale for the adjuvant treatment of heart failure patients with Coenzyme Q10 supplements [22].
Briefly, the New York Heart Association classes, widely used in the diagnosis of heart failure patients, describe patients as follows:
- Class I: feeling capable of ordinary physical exertion but showing signs of early fatigue and shortness of breath at higher levels of exertion
- Class II: feeling comfortable at rest but showing signs of early fatigue and shortness of breath and discomfort at ordinary levels of physical activity
- Class III: feeling fatigue, heart palpitation, or shortness of breath during light activity
- Class IV: feeling fatigue, heart palpitation, or shortness of breath even while at rest
1980s: Folkers and Mortensen: Supplementation of heart disease patients with Coenzyme Q10 in open-label studies
Dr. Mortensen and Dr. Folkers started slowly, testing the effects of Coenzyme Q10 supplementation in heart disease patients in open-label trials, studies in which both the researchers and the patients knew who was getting the active substance and when he was getting it [66]. The researchers gave 100 milligrams of Coenzyme Q10 daily to patients with advanced heart failure. All of the patients had been showing an unsatisfactory response to the conventional medical treatment using diuretics and digitalis.
The researchers followed the progress of the patients receiving the Coenzyme Q10 supplementation for seven months. When the patients were on the Coenzyme Q10, their symptoms – early fatigue, shortness of breath – improved. When the Coenzyme Q10 treatments were discontinued, the patients suffered relapses. This study provided some of the first empirical evidence of the effectiveness of Coenzyme Q10 as an adjuvant therapeutic agent in advanced cases of heart failure.
The management of chronic heart failure (Folkers and Mortensen)
By 1990, Dr. Mortensen and Dr. Folkers had amassed enough clinical evidence from blood samples, heart biopsy tissue samples, and measurements of cardiac function that they could publish a list of clinical benefits of Coenzyme Q10 supplementation of heart failure patients with corresponding biochemical correlates, all of which suggested, in their words, a scientific breakthrough in the management of chronic heart failure [65].
- They had seen the significant inverse association between the level of blood and tissues concentrations of Coenzyme Q10 and the severity of heart failure.
- They had seen significant improvement in the symptoms and survival of heart failure patients treated with 100 milligrams of Coenzyme Q10 daily.
- They had seen relapses whenever the administration of Coenzyme Q10 to heart failure patients was discontinued.
1985: Langsjoen and Folkers: Double-blind study of the effect of Coenzyme Q10 on cardiomyopathy
In 1985, Dr. Per Langsjoen of Tyler, Texas, and Dr. Folkers published the results of double-blind studies showing statistically significant beneficial effects of supplementation with Coenzyme Q10 [47,48]. The researchers enrolled 19 of Dr. Langsjoen’s class III and class IV heart failure patients in a placebo-controlled, double-blind, cross-over study. All of the 19 patients had low or borderline concentrations of Coenzyme Q10 in their blood. All of the patients showed a statistically significant increase in their blood CoQ10 levels during the period of supplementation with the active substance (and not in the period with the placebo substance).
One group of patients received first Coenzyme Q10 for 12 weeks and, then, after a washout period, placebo for 12 weeks. The other group received first placebo for 12 weeks, and, then, after a washout period, Coenzyme Q10 for 12 weeks. The researchers monitored the blood levels of Coenzyme Q10 and aspects of cardiac function at points 0, 4, 16, and 28 weeks.
In these patients, who would normally have been expected to die within two years’ time while on conventional heart disease medications, the researchers measured significant improvements in various aspects of cardiac function and patient well-being associated with the concomitant increases in blood Coenzyme Q10 levels during the periods of active treatment. In particular, the researchers recorded significant improvements in the following parameters:
- stroke volume (the amount of blood pumped out in a single contraction of the heart)
- ejection fraction (the percentage of the blood being pumped out of the heart as it contracts)
Drs. Langsjoen and Folkers attributed the significant improvement to the role of Coenzyme Q10 in the bioenergetics in the heart muscle cells. Coincidentally, 1985, the year of the publication of the double-blind study results by Dr. Per Langsjoen and Dr. Folkers was the year that Dr. Langsjoen’s son Dr. Peter H. Langsjoen began his career in cardiology.
1988: Langsjoen and Folkers: Blood Coenzyme Q10 levels of 2.5 mg/L or higher to improve symptoms in heart failure
Dr. Langsjoen and Dr. Folkers compiled the data from 115 heart failure patients. 88 of the patients completed the course of therapy with Coenzyme Q10 [49]. The patients were monitored for the following parameters: ejection fraction, cardiac output, and NYHA functional classification. Nearly 80% of the patients showed significant improvements in two of the three parameters.
The patients with the lowest baseline ejection fractions showed the highest increases, but also those patients with higher baseline ejection fractions showed increases with the Coenzyme Q10 therapy.
There were also significant improvements in NYHA classifications: 17 of 21 patients in class IV, 52 of 62 patients in class III, and 4 of 5 patients in class II improved their status to a lower functional class.
Dr. Langsjoen concluded that the patients’ clinical responses to therapy with Coenzyme Q10 appeared to be best when the supplementation raised the blood Coenzyme Q10 levels to approximately 2.5 micrograms/mL (2.5 mg/L) or higher.
1990: Langsjoen and Folkers: Six-year clinical study of Coenzyme Q10 supplementation
In 1990, Dr. Langsjoen (father), Dr. Langsjoen (son), and Dr. Folkers published a six-year clinical study of successful therapy of cardiomyopathy with Coenzyme Q10 supplementation in 143 heart failure patients in NYHA classes III and IV [50].
Langsjoen and Littarru: Concerns about the relationship between statin medications and atherosclerosis and heart failure
Already in 1990, Dr. Folkers and the elder Dr. Langsjoen had published research results showing that the use of the statin medication lovastatin decreases Coenzyme Q10 levels in humans [23]. Later, in 2007, together with Dr. Gian Paolo Littarru, the younger Dr. Langsjoen published a warning about a possible causal connection between the use of statin medications and the stimulation of atherosclerosis and heart failure [57]. More on this topic later in this history.

Dr. Peter H. Langsjoen, a cardiologist much concerned about the effect of statin medications on his patients
1980s: Judy and Folkers: Independent confirmation of the Folkers/Mortensen and the Folkers/Langsjoen results by Judy and Folkers
In the same period of the 1980s, Dr. William V. Judy was monitoring the progress of heart failure patients at Methodist Hospital and at St. Vincent Hospital in Indianapolis, Indiana.
Dr. Judy reported significant improvement in heart failure symptoms and survival in studies involving 34 patients with NYHA class IV heart failure and involving 180 patients with NYHA class III and IV heart failure when the administration of Coenzyme Q10 was added to the conventional regimen of heart failure medication [38]. Moreover, Dr. Judy observed and reported on the same sort of clinical relapse and worsening of the congestive heart failure that Dr. Mortensen had reported whenever the administration of Coenzyme Q10 was discontinued.
Dr. Judy reported improvement in cardiac function in patients treated with Coenzyme Q10 as compared with patients receiving placebo:
- improved cardiac index (heart output as it relates to body size)
- improved left ventricular ejection fraction (% of blood pumped)
- reduced left ventricular end-diastolic volume (volume of blood in the chamber at end-load)

Dr. William V. Judy, former Indiana University professor of physiology and bio-physics, Coenzyme Q10 researcher, and founder of SIBR Research Institute
1986: Dr. Folkers awarded the Priestley Medal
In 1986, the American Chemical Society honored Dr. Folkers with a Priestley Medal, the highest honor that the ACS can confer on a chemist. By that time, Dr. Folkers was the world’s leading Coenzyme Q10 researcher. He and his cardiologist collaborators had established a biochemical rationale for the administration of Coenzyme Q10 to heart failure patients.
1990: President George H. W. Bush awarded Dr. Folkers the National Medal of Science in recognition of Dr. Folkers’ contributions to the advancement of knowledge in chemistry.

President George H. W. Bush congratulates Dr. Karl Folkers on being awarded the National Medal of Science for chemistry.
Explanations for Coenzyme Q10 deficiency in heart muscle cells
Dr. Folkers and the cardiologists thought that the possible explanations for the Coenzyme Q10 deficiency in the heart muscle cells leading to the development and worsening of heart failure might be the following reasons:
- Decreased bio-synthesis of Coenzyme Q10 with increasing age
- Increased demand for Coenzyme Q10 caused by various neurohormonal responses
- Inhibition of Coenzyme Q10 bio-synthesis caused by drug interactions
- Low intakes of Coenzyme Q10 from food
- “Steal phenomenon” – alterations in blood flow patterns causing oxidative stress of such magnitude in the failing heart that the available Coenzyme Q10 is used more and more as an antioxidant
The Coenzyme Q10 situation by 1990
In 1990, then, Coenzyme Q10 became available as a dietary supplement in the United States and in Europe. Until that time, Coenzyme Q10 had been available as a prescription medical drug in Japan and as a research drug in the United States and Europe.
By the time that the 1990s rolled around, Dr. Folkers himself and Dr. Littarru and Drs. Mortensen, Langsjoen, and Judy were convinced of the need for the inclusion of adjunctive treatment with Coenzyme Q10 in the guidelines for the treatment of chronic heart failure patients. They thought that their clinical trial data represented a break-through.
Adequate tissue concentrations of Coenzyme Q10 are necessary for the proper bio-energetic functioning of the heart muscle. The medical community, however, wanted more clinical trial evidence before it was willing to consider changing the guidelines for the treatment of chronic heart failure.
Further considerations: Coenzyme Q10 as an antioxidant
By the 1990s, Dr. Folkers was well aware of the antioxidant activity of the Coenzyme Q10 molecule. Already in 1977, the Swedish researcher Dr. Lars Ernster had published work on the importance of Coenzyme Q10 as an antioxidant and scavenger of harmful free radicals [18]. Dr. Folkers hypothesized that the antioxidant form of Coenzyme Q10 might enhance the effectiveness of chemotherapy drugs. He also thought that antioxidants such as Coenzyme Q10 might be useful in suppressing the activity of certain tumor-associated cytokines that otherwise help to maintain the growth of tumors.
Moreover, as Coenzyme Q10 was known to be a very safe and affordable oral supplement, Dr. Folkers saw no reason why it should not be added, experimentally, to anti-tumor medication regimens. Hodges, Hertz, Lockwood, and Lister present a more complete explanation of Dr. Folkers’ thinking in their 1999 BioFactors review article [31].
1992: Stocker and Coenzyme Q10 as a potent antioxidant
Dr. Roland Stocker and research associates in Australia demonstrated that oral supplementation with Coenzyme Q10 in the ubiquinone form increases the concentration of Coenzyme Q10 in its reduced form, ubiquinol, in the plasma and in all lipoproteins [63]. Supplementation with Coenzyme Q10 thereby increases the resistance of the low-density lipoproteins to harmful radical oxidation.
Dr. Stocker showed that a single oral dose of 100 milligrams or 200 milliggrams of Coenzyme Q10 in the form of ubiquinone increased the total Coenzyme Q10 content in plasma by 80% or 150%, respectively, within 6 hours [63]. Longer-term supplementation (100 milligrams Coenzyme Q10 three times a day) resulted in a fourfold increase of ubiquinol in the plasma and the LDL.
To test the role of Coenzyme Q10 as an antioxidant, Dr. Stocker and his colleagues induced oxidation of the low-density lipoproteins using a mild, steady flow of aqueous peroxyl radicals, which resulted immediately in a very slow formation of lipid hydroperoxides. In each case, the rate of the induced lipid oxidation increased noticeably whenever 80%-90% of the ubiquinol had disappeared. Dr. Stocker noted that the amount of the dose of aqueous peroxyl radicals needed to reach the breaking point in the lipid oxidation was proportional to the amount of ubiquinol already incorporated in the low-density lipoproteins (Mohr).
The significance of this early work by Dr. Stocker to cardiovascular disease is the belief that oxidative modification of low-density lipoproteins is a crucial step in the development of atherosclerosis (the build-up of plaques of fatty materials on the inner walls of the arteries). In addition to improving the bioenergetics of the heart muscle, supplementation with Coenzyme Q10 can reduce the extent of damaging oxidative modification of the low-density lipoproteins.
1993: The Morisco multi-center heart failure study
There were also encouraging results from Italy. Italian researchers published the results of a randomized, double-blind, placebo-controlled study of 641 patients classified as NYHA class III or IV [64]. The researchers believed that mitochondrial dysfunction and energy starvation in the heart muscle are what cause congestive heart failure. They tested the hypothesis that Coenzyme Q10 adjunctive treatment could ameliorate the symptoms of heart failure.
The Italian patients received two milligrams of Coenzyme Q10 per kilogram of body weight per day for a year. That meant that an average 160-pound man received about 150 milligrams of Coenzyme Q10 per day. The researchers found that Coenzyme Q10 supplementation, when added to conventional therapy, resulted in significantly fewer cases of pulmonary edema and cardiac asthma, significantly fewer serious complications, and significantly fewer hospitalizations. In many respects, the results of the Morisco study foretold the results of the Q-Symbio study.
1993: The Baggio multi-center heart failure study
The results of the largest clinical trial to date – the Italian multi-center study enrolling 2664 heart failure patients in NYHA classes II and III in an open-label study conducted in 173 Italian heart centers – were also published in 1993 [8]. To the conventional treatment with digitalis, diuretics, and vasodilators, the researchers added a daily dose of Coenzyme Q10 in the range from 50-150 milligrams. Most patients (78%) received 100 mg/day.
The Italian researchers regarded heart failure as a condition of energy depletion in the heart muscle, and they tested the clinical efficacy of Coenzyme Q10 adjunctive treatment in heart failure. They evaluated clinical parameters upon patients’ entry into the study and then again after three months. At the end of the study, three out of every four patients showed improvement in one or more of the following symptoms:
- Cyanosis (a sign of insufficient oxygen in the blood)
- Edema (excessive accumulation of fluid in body cavities)
- Pulmonary rales (rattling sounds caused by lung congestion)
- Jugular reflux (a sign of distension of the jugular vein)
- Palpitations (irregular heart beat)
- Perspiring
- Vertigo (loss of balance)
54% of the patients showed an improvement in at least three symptoms; the researchers regarded this to be a sign of improved quality of life.
In addition, five out of every eight patients showed an improvement in arrhythmia. Less than one percent of the participants in the three-month-long study experienced any side effects, and not all of those side effects – nausea, gastrointestinal disturbance, rash – could be linked to the Coenzyme Q10 treatment.
Mid-1990s: Dr. Folkers ready to move on to cancer research
At some point in the mid-1990s, Dr. Folkers decided that he was sufficiently convinced by the available evidence. Coenzyme Q10 supplementation added on to conventional medical therapy does significantly improve the cardiac function and the quality of life and survival of heart failure patients. Dr. Folkers was ready, he said, to move on to research involving Coenzyme Q10 and cancer patients.
Dr. Folkers’ thinking on the subject of Coenzyme Q10 and the treatment of cancer was that, first of all, adequate supplies of Coenzyme Q10 are necessary for normal cell respiration and functioning. Abnormally low levels of Coenzyme Q10 in the cells could conceivably disrupt the normal functioning of the cells, could result in abnormal patterns of cell division, and could possibly result in the development of tumors.
Wanting to build on the research of Dr. Emile Bliznakov
In the back of his mind, Dr. Folkers was remembering the animal studies done by another of his early collaborators, Dr. Emile Bliznakov. Using laboratory mice for whom Coenzyme Q9 is the predominant Coenzyme Q, Dr. Bliznakov had shown a number of thought-provoking results:
- Administration of small dosages of Coenzyme Q10, ranging from 150 to 750 micrograms, to laboratory mice improved phagocyte activity and increased antibody counts in the mice. Phagocytes are the immune system cells that engulf and absorb harmful foreign microorganisms before they can hurt the cells and tissues.
- Administration of Coenzyme Q10 to the mice delayed the onset of tumor growth, limited the growth of tumors, and reduced mortality when Dr. Bliznakov induced tumor growth by injecting a carcinogen.
- Administration of Coenzyme Q10 reduced the mortality rate in laboratory mice infected with leukemia virus.
Basically, Dr. Bliznakov had demonstrated, in animals, that there is a positive association between aging and depressed Coenzyme Q10 levels and depressed immune response and that Coenzyme Q10 supplementation boosts immune response.
1990s: Swedish and American human cancer studies
Studies of human cancer patients in both Sweden and the United States had revealed abnormally low levels of blood Coenzyme Q10 concentrations in patients suffering from breast, lung, and pancreas cancer. Dr. Folkers was impatient to initiate augmentative Coenzyme Q10 treatment to cancer patients.
1990s: The Folkers and Lockwood breast cancer study
During a five-year period in the 1990s, in close collaboration with Dr. Folkers and Sven Moesgaard, the Danish doctor Knud Lockwood treated 32 women with breast cancer with a therapeutic formulation that included 390 milligrams of Coenzyme Q10 daily – a high level at that time – and various antioxidant vitamins and minerals and omega-3 and omega-6 fatty acids [58,59]. Eli Wallin and Sven Moesgaard of Pharma Nord provided the Coenzyme Q10 and the other antioxidants and polyunsaturated fatty acids used in the breast cancer study.

Left to right: Sven Moesgaard, Dr. Karl Folkers, and Dr. Knud Lockwood, the authors of the ANICA (Antioxidant Nutritional Intervention in CAncer) breast cancer study
1990s: Partial and complete remissions and extended survival
Dr. Lockwood treated the 32 high-risk breast cancer patients whose cancer had spread to their lymph nodes with the antioxidant nutritional supplementation added on to the conventional protocol of breast cancer therapy such as Tamoxifen [59].
The results of the Coenzyme Q10 and antioxidant adjunctive treatment can be summarized in the following way:
- No patients died during the study period when the expected number of deaths was four
- No patients showed any signs of additional distant tumors
- The patients’ quality of life was improved; the patients did not lose weight, and they reduced their use of pain medications
- Six patients showed partial or complete remission
Need for randomized controlled trial of Coenzyme Q10 and cancer
The ANICA breast cancer study was an open-label trial without a proper control group. Its sample size was small. Dr. Folkers was determined to find funding for a randomized controlled trial of adjunctive Coenzyme Q10 treatment for cancer patients. Such a clinical trial would have focused not only on remission and survival but also on quality of life and the lessening of the adverse side effects of anti-cancer drugs. It would have tested the use of even higher daily dosages of Coenzyme Q10.
Coenzyme Q10 and prostate cancer
The last cancer management study that Dr. Folkers participated in was a study of the effect of the treatment of prostate cancer with Coenzyme Q10. He did a series of three open-label prostate cancer studies in collaboration with Dr. William Judy. The use of Coenzyme Q10 was positively associated with stopping the growth and the spread of prostate cancer. After about 120 days of Coenzyme Q10 supplementation with a dosage of 600 mg/day, plasma PSAs and prostate mass decreased significantly. The younger patients appeared to be more responsive than the older patients. In 1998, Dr. Judy presented the results of the prostate cancer studies at the American College for Advancement in Medicine (ACAM) meeting in Fort Lauderdale, Florida.
Dr. Folkers was so excited by the results of the ANICA study and the prostate cancer studies that he went to Denmark, Sweden, and Finland to find the funding to continue these studies. With much assistance from Sven Moesgaard and the Swedish researcher Magnus Nylander, Dr. Folkers spent the final year of his life in 1997 trying to set up cancer research protocols in Denmark, Sweden, and the United States.

Left to right: Dr. Josef Mainz, Dr. Magnus Nylander, Sven Moesgaard, Dr. Svend Aage Mortensen
Cardio-toxicity of cancer drugs and the role of Coenzyme Q10
As early as the 1980s, Japanese researchers had seen toxic effects of the cancer drug Adriamycin (doxorubicin) on the heart muscle. They had noticed that patients taking an adjuvant Coenzyme Q10 therapy suffered less damage to the heart muscle.
In 1984, Dr. Judy and Dr. Folkers and a team of researchers did a study in which they investigated the effects of Coenzyme Q10 adjunctive treatment in lung cancer patients who were being treated with Adriamycin [40]. The treatment group received the Adriamycin plus Coenzyme Q10. The control group received Adriamycin and a placebo.
The Coenzyme Q10 treatment group was able to take twice as much Adriamycin with little or no evidence of cardio-toxicity. The control group had a significant loss in cardiac ejection fraction and significant left ventricular dysfunction. Participants in the control group, the group not being supplemented with Coenzyme Q10, had to stop taking Adriamycin because of worsening heart failure.
1990: Pharma Nord enters the history of Coenzyme Q10
Dr. Mortensen was not the only person in Denmark whom Dr. Folkers was urging to do more and better research. He was also pushing Sven Moesgaard of Pharma Nord to do research into the absorption, the bio-availability, and the health effects of Pharma Nord’s Bio-Quinone Q10 product.
In the city of Vejle, Denmark, Eli Wallin and Sven Moesgaard had established the firm that they called Pharma Nord. Their first product, launched in 1984, was a Bio-Selenium and Zinc preparation, consisting of 100 micrograms of organic selenium and 15 milligrams of zinc.

Left to right: Eli Wallin, Karl Folkers, Sven Moesgaard. At Pharma Nord, Eli Wallin served as Administrative and Financial Director, and Sven Moesgaard served as Research and Technical Director.
Selenium was an important nutritional supplement in northern Europe, which has generally selenium-poor soils and selenium-poor foods. Selenium is a co-factor in several important antioxidant enzymes, it regulates thyroid function, and it may help to reduce the risk of various cancers. Zinc is also a component in many enzyme activities and helps to strengthen the immune system.
Moreover, there seems to be an important synergistic relationship between Coenzyme Q10 and selenium in the body. Selenium deficiencies can inhibit the cells from getting optimal concentrations of Coenzyme Q10, and, adequate concentrations of Coenzyme Q10 must be available for the cells to benefit from optimal selenium function [5].
1990s: Pharma Nord’s Bio-Quinone Q10
The crystalline Coenzyme Q10 in raw material form will not completely dissociate to single molecules in a lipid at body temperature. The crystalline Coenzyme Q10 raw material will completely dissociate to single Coenzyme Q10 molecules in a lipid only at a temperature of 10 degrees centigrade above body temperature. Because humans cannot absorb Coenzyme Q10 crystals and cannot live with a body temperature of 47 degrees centigrade, producers of Coenzyme Q10 capsules must necessarily use a heat treatment on the Coenzyme Q10 raw material to dissolve the crystalline raw material.
Realizing the central importance of Coenzyme Q10 in the process of cellular bioenergetics and in the antioxidant protection of the cells, the directors of Pharma Nord began to experiment to find the best way to take the yeast-fermented Coenzyme Q10 raw material and dissolve it in oils to make it more easily absorbed. Coenzyme Q10 is, after all, a highly fat-soluble substance, its crystals need a higher temperature than body temperature to dissolve, and the absorption cells in the small intestine cannot absorb crystals. The formulation of the Coenzyme Q10 preparation is not easy to get right.
Eli Wallin and Sven Moesgaard wanted to sell only those products that they themselves wanted to take, and they had no interest in taking a nutritional supplement with a poor absorption rate. So, they knew that they had their work cut out for them. Even before they could think about doing studies of the health effects of Bio-Quinone, they needed to do absorption and bio-availability studies.

Sven Moesgaard and Eli Wallin of Pharma Nord – making products that they themselves wanted to take based on solid research results
1990s: Coenzyme Q10 absorption and bioavailability studies
The concept of absorption in the context of the oral supplement Coenzyme Q10 refers to the amount of Coenzyme Q10 that passes from the mouth to the stomach to the small intestine and through the absorption cells of the small intestine into the lymph and then into the blood. Typically, after absorption, the ingested Coenzyme Q10 passes slowly through the lymph and reaches a peak concentration in the blood between 5 and 8 hours later. It is only from a single-dose study that the percentage of the ingested dose can be used to calculate the percentage of the dose that has been absorbed.
Bioavailability is generally defined as the degree to which or the rate at which a substance is absorbed or becomes available at the site in the body where it exerts its physiological activity. The concept of bioavailability refers to the accumulation (storage) of the Coenzyme Q10 in the blood over time. Bioavailability is typically measured at 7, 14, 21, and 30 days over an interval of extended supplementation with a set daily dosage.
Absorption of Coenzyme Q10 is in no way close to 100 % of the ingested dose. The crystalline (dry powder) Coenzyme Q10 forms generally have an absorption of less than one percent. Dry powder Coenzyme Q10 suspended in oils generally has an absorption between 1.5 and 3.0 percent. The crystal-free Coenzyme Q10 products generally have an absorption at Cmax of 5 to 8 percent.
By contrast, injected substances (not Coenzyme Q10) will have 100 percent bioavailability. However, the nutritional supplement Coenzyme Q10 is taken orally. As such, it will not have the 100 percent bioavailability. Oral Coenzyme Q10, because of the difficulties involved in its absorption, falls far short of 100 percent bioavailability.
1990s: Bioavailability of Pharma Nord’s Bio-Quinone Q10
In 1994, Dr. Folkers and Sven Moesgaard published the results of a one-year bioavailability study of Bio-Quinone Q10 [25]. The researchers gave 21 healthy participants 30 milligrams of Coenzyme Q10 three times a day for nine months. There then followed a withdrawal period of three months. The researchers took blood samples from the participants before the start of the supplementation, after three months and nine months of supplementation, and, again, three months after the withdrawal of supplementation.
The supplementation with Bio-Quinone Q10 increased the mean blood Coenzyme Q10 concentration from about 1 mg/L before supplementation to about 2 mg/L after three and nine months of supplementation. The mean blood Coenzyme Q10 levels dropped back down to the pretreatment levels after withdrawal. The increase in the blood Coenzyme Q10 concentration was statistically significant.
In a second study published in 1994, Sven Moesgaard and Malene Weis and Dr. Mortensen and a team of researchers investigated the bioavailability of four different Coenzyme Q10 preparations in a randomized, four-way, cross-over study [90]. Their results showed that the soybean oil formulation used at the time in Pharma Nord’s Bio-Quinone Q10 preparation yielded a bioavailability superior to that achieved by three different formulations.
Then, in 1997, Danish researchers investigated the absorption of dietary Coenzyme Q10 ingested either as a single 30-milligram dose of Bio-Quinone Q10 or as a meal of cooked pork heart containing 30 milligrams of Coenzyme Q10 [89]. Both methods significantly raised the serum Coenzyme Q10 levels in the study participants. There was no significant difference between the increases in absorption of the two methods.
1990s: Pharma Nord researching Coenzyme Q10 from the beginning
The point of listing and summarizing the above early absorption and bioavailability studies is to show that Pharma Nord is a company that has been around from the beginning and has been willing to do the research. In this, the influence of Dr. Folkers and Dr. Mortensen on Eli Wallin and Sven Moesgaard can be seen very clearly.

Sven Moesgaard and Dr. Karl Folkers (right) – Dr. Folkers met frequently with Sven Moesgaard and constantly urged him to do more research on the absorption and effects of Coenzyme Q10
1990s: Pharma Nord research on the effects of Coenzyme Q10
Pushed by Dr. Folkers, Pharma Nord did more research on the effects of Coenzyme Q10 supplementation than any other producer. In the 1990s alone, under the guidance of Sven Moesgaard, the company achieved research results that are relevant even today:
- Demonstrated that patients diagnosed with type-1 diabetes can take Coenzyme Q10 without risk of hypoglycemic episodes (Henriksen) [29]
- Demonstrated that Coenzyme Q10 supplementation is well tolerated by elderly type-2 diabetes patients and that their glycemic control is not affected by the supplementation (Eriksson) [19]
- Demonstrated that supplementation with 100 milligrams of Coenzyme twice daily improves left ventricular performance in patients diagnosed with chronic heart failure (Munkholm) [73]
- Demonstrated that supplementation with 100 milligrams of Coenzyme Q10 daily significantly reduces the extent of gingival bleeding in patients diagnosed with periodontitis (Nylander) [74]
- Demonstrated that Coenzyme Q10 supplementation can reduce gingival inflammation (Denny) [15]
- Demonstrated that supplementation with 90 milligrams of Coenzyme Q10 daily significantly improves measured indexes of physical performance in top-level cross-country skiers (Ylikoski) [91]
- Demonstrated that supplementation with Coenzyme Q10 may be beneficial in improving sperm motility (Lewin) [56]
- Demonstrated evidence that 90 milligrams of Coenzyme Q10 supplementation daily has an antioxidative effect in the blood where there are many lipids vulnerable to peroxidation (Weber) [88]
- Demonstrated that supplementation with Coenzyme Q10 may protect the heart from ischemia/reperfusion injury (Yokoyama) [92]
- Demonstrated that supplementation with Coenzyme Q10 improves the quality of life of breast cancer patients (no more loss of weight, reduced use of pain medications, no additional metastases) (Lockwood) [58,59]
- Demonstrated that daily supplementation with 100 milligrams of Coenzyme Q10 and 100 micrograms of selenium benefits acute myocardial infarction patients (Kuklinski) [46]

Dr. Bodo Kuklinski, Director of the Diagnostic and Therapeutic Center for Environmental Medicine, Rostock, Germany – one of the first researchers to use Coenzyme Q10 and selenium supplements to treat heart disease patients
1994: Energy and Defense by Dr. Gian Paolo Littarru
In 1994, Dr. Littarru, a professor of biochemistry at the University of Ancona Medical College in Ancona, Italy, published a book entitled Energy and Defense. The book’s subtitle was Facts and Perspectives on Coenzyme Q10 in Biology and Medicine. The word Energy in the title refers to the bioenergetics role of Coenzyme Q10 in the cells, and the word Defense in the title refers to the antioxidant role of Coenzyme Q10 in quenching harmful free radicals and protecting the body from oxidative damage.
In his introduction, Dr. Littarru remembered and paid tribute to the pioneers in Coenzyme Q10 research: Dr. R. A. Morton, Dr. Fred Crane, Dr. Karl Folkers, Dr. Peter Mitchell, Dr. Yuichi Yamamura, and Dr. Per Langsjoen. Dr. Svend Aage Mortensen wrote the text on the inside of the book jacket.

For many years, Dr. Littarru has been a professor teaching biochemistry to medical students. Until recently, he has served as the chairman of the International Coenzyme Q10 Association. His primary research interest has always been biomedical research on Coenzyme Q10.
1997: Statins lower serum Coenzyme Q10 concentrations
Dr. Mortensen and a team of researchers enrolled 45 hypercholesterolemia patients in a randomized, double-blind study [69]. The patients were treated with increasing dosages of either lovastatin (20-80 mg/day) or pravastatin (10-40 mg/day) over a period of 18 weeks. The researchers measured serum levels of Coenzyme Q10 and cholesterol at baseline and at the end of the study.
They found significant cholesterol-dose-related declines in the serum concentrations of Coenzyme Q10 in both the pravastatin group and the lovastatin group. Dr. Mortensen concluded that the cholesterol inhibiting statin medications are safe and effective in the short run; however, he also noted that there is a need to monitor patients to see if the lowering of Coenzyme Q10 becomes increasingly important during long-term use of statin medications.
1997: Soja and Mortensen: The first meta-analysis of Coenzyme Q10 and heart failure research
In 1997, one of Dr. Mortensen’s graduate students at Copenhagen University published a meta-analysis of the treatment of congestive heart failure with Coenzyme Q10 in eight clinical trials conducted in the period from 1984 to 1994 [87]. The results of the meta-analysis showed that adjunctive treatment of heart failure patients with Coenzyme Q10 significantly improved the following parameters:
- stroke volume
- cardiac output
- ejection fraction
- cardiac index
- end diastolic volume index
- systolic time intervals
- total work capacity
The beneficial effects of Coenzyme Q10 as an adjunctive treatment of heart failure were beginning to be well-documented.
1997: The death of Dr. Folkers
On December 9, 1997, having recently flown back to the United States from Sweden, Dr. Folkers died of a heart attack caused by a blood clot. In Sweden, he had been trying to set up clinical studies of the efficacy of Coenzyme Q10 in the treatment of cancer patients.
For many years, Dr. Folkers had served as the editor of the proceedings of the International Symposium on Coenzyme Q published by Elsevier, Inc. in Amsterdam. The symposium proceedings were published under the title Biomedical and Clinical Aspects of Coenzyme Q, Upon Dr. Folkers’ death, Dr. Gian Paolo Littarru took over as editor of the symposium proceedings.
1997: The founding of the International Coenzyme Q10 Association
Also in 1997, Dr. Gian Paolo Littarru, Dr. Svend Aage Mortensen, and Sven Moesgaard were the driving force behind the founding of the nonprofit organization, The International Coenzyme Q10 Association.
Article 3 of the Statutes of the Association states that the purpose of the Association is to promote basic and applied research on the biomedical aspects of Coenzyme Q10 in order to diffuse knowledge on basic biochemistry and genetics, and on the preventive and/or therapeutic effects of Coenzyme Q10.
For many years, Pharma Nord’s Myoqinon preparation has been the preparation selected by International Coenzyme Q10 Association members for use in research studies. The dietary supplement edition of Myoqinon is Bio-Quinone Active CoQ10 GOLD. The quality of those two preparations are identical
During the period October 11 – 15, 2016, the International Coenzyme Q10 Association held its 8th international conference. The conference, organized by Professor Dr. Giorgio Lenaz, Dr. Maria Luisa Genova, Professor Dr. Anna Ida Falasca and Professor Dr. Plácido Navas, was held at the University of Bologna in Italy. Dr. Plácido Navas,
Professor of Cell Biology at the Universidad Pablo de Olavide-CSIC in Sevilla, Spain, is the present president of the Coenzyme Q10 Association (website: www .icqa.org).
1998: Coenzyme Q10 in patients with acute myocardial infarction
As the 20th century drew to a close, Singh and Chopra reported on the results of a randomized, double-blind, placebo-controlled trial of Coenzyme Q10 in patients with acute myocardial infarction [85]. For 28 days, the researchers gave 73 patients 120 mg/day and gave 71 patients a placebo preparation.
The Coenzyme Q10 treatment yielded significant improvements in angina pectoris, total arrhythmias, and left ventricular function. Moreover, the Coenzyme Q10 treatment was associated with significantly lower cardiac events such as cardiac death and nonfatal second heart attacks. There was a greater reduction in lipid peroxides, indicators of oxidative stress, in the treatment group than in the placebo group. The study results indicate that supplementation with Coenzyme Q10 can provide protective benefits in patients with acute myocardial infarctions if the Coenzyme Q10 is administered within 3 days of the onset of symptoms.
Looking ahead to the 21st Century: The Guidelines
As he looked forward to the 21st century, Dr. Mortensen’s goal was to convince the American College of Cardiology and the American Heart Association of the need to amend the guidelines for the treatment of heart failure to include adjuvant treatment with Coenzyme Q10 [1]. He thought that energy starvation of the heart muscle cells is a dominant feature of the heart failure condition.
The link between a deficiency of Coenzyme Q10 in blood and tissue and the severity of heart failure had been established. Coenzyme Q10 was a logical adjunct treatment for heart failure patients. It had only very seldom side effects, which, if they occurred, were mild. Several randomized controlled trials enrolling altogether more than 1000 heart failure patients had shown that Coenzyme Q10 adjuvant treatment positively affects clinical parameters, lowers NYHA class, improves exercise capacity, and reduces the need for hospitalization [68].
The beginning of the 21st century of Coenzyme Q10 research
The 21st century in Coenzyme Q10 research started slowly. The really big randomized controlled trials, the KiSel-10 study [3] and the Q-Symbio study [70], were still in the planning stage. But there were some interesting results, nonetheless, in the first years of the 2000s.
2002: Engelsen: Coenzyme Q10 safe for warfarin patients
One question that remained to be answered was the question of whether Coenzyme Q10 is safe to use in patients taking the anti-coagulant Coumadin (warfarin). Engelsen and a team of Danish researchers tested the effect of a daily dosage of 100 milligrams of Coenzyme Q10 for four weeks on 24 patients who were on long-term warfarin medication [17]. The patients’ international normalized ratio (INR) remained stable throughout the treatment period. The mean dosage of the warfarin treatment did not change during the treatment period; 36.5 mg/week (29.1-45.8). The researchers concluded that Coenzyme Q10 does not influence the clinical effect of warfarin.
A couple of years later, Zhou (2005) reported research results in rats that indicated that supplementation with Coenzyme Q10 did not have an effect on the absorption and distribution of warfarin but did produce a significant increase in the total serum clearance of warfarin [93].
As a matter of caution, patients taking an anti-coagulant should consult with the prescribing physician before taking Coenzyme Q10 supplements. The Coenzyme Q10 could make hitting anticoagulation targets more difficult, and that is difficult enough as it is.
2003: Mortensen: Preparing for the multinational Q-Symbio clinical trial
Dr. Mortensen began to prepare the bio-chemical rationale and the design and the end-points for a multinational clinical trial: the Q-SYMBIO clinical trial, a study with focus on the SYMptoms, BIomarker status, and long-term Outcomes (notably hospitalizations and mortality) of supplementation with Coenzyme Q10 [68,70].
2003: Dr. Mortensen’s review of the existing double-blind studies
The first thing Dr. Mortensen did was review the existing 13 well-designed studies, the randomized, double-blind, placebo-controlled studies [68].
- Taken together, the studies comprised the results from over 1000 patients with heart failure
- All of the studies had a cross-over design or a parallel groups design
- All of the studies but one used between 100 and 200 milligrams of Coenzyme Q10 per day
- None of the studies reported any significant side effects
- Ten of the 13 studies showed positive effects of adjunctive treatment of heart failure patients with Coenzyme Q10: improvements in symptoms, exercise capacity, and quality of life
- Three of the 13 studies had neutral outcomes
- The improvement in exercise capacity associated with Coenzyme Q10 treatment had the same order of magnitude as the improvement in exercise capacity associated with the use of ACE inhibitors in heart failure patients
2003: Alehagen: the first elderly participants enrolled in KiSel-10 study
Dr. Urban Alehagen and researchers at the University Hospital, Linköping, Sweden, began enrolling elderly Swedish citizens in the KiSel-10 clinical trial [3]. KiSel-10 was a study of the effect of a combined intervention with selenium (SelenoPrecise® 200 micrograms/day) and Coenzyme Q10 (Bio-Quinone Q10 100 milligrams twice a day) on cardiovascular mortality and cardiac function in the elderly population in the Kinda municipality in southeastern Sweden.
Ki in the study name KiSel-10 stands for the Kinda municipality in Sweden, Sel stands for the organic SelenoPrecise® selenium, and 10 stands for the Myoqinon Q10 in vegetable oil.
Myoqinon is the pharmaceutical edition of Bio-Quinone GOLD. It is registered in an EU country and is used in scientific studies. SelenoPrecise is also registered in the EU, both in a pharmaceutical edition and as a dietary supplement
The researchers enrolled elderly persons aged 70 – 88 years who could be expected to fulfill a study period of five years. The researchers enrolled 443 participants but excluded any elderly individuals who met any of the following exclusion criteria:
- Recent heart attack (within four weeks)
- Any cardiovascular operative procedure planned within the next four weeks
- Inability to consent to participate in the study or to understand the consequences of participation
- Any evidence of a serious disease that would reduce the chance of survival or make it impossible to complete the full five-year study period
- Long and difficult transport to the primary health center
- Drug or alcohol abuse
The following considerations motivated the researchers:
- Many Northern Europeans have low serum selenium levels
- Selenium is used by the cells to build approximately 25 different enzyme systems in the body
- The cells need the presence of Coenzyme Q10 to produce selenium-containing enzymes
- A combined intervention of the two supplements would increase serum concentrations in elderly citizens sufficiently to have a significant effect on mortality and cardiac function
The intervention period for each enrolled participant was to be 4 years. Blood samples and cardiac natriuretic peptide levels were analyzed at the beginning, at every six months, and at the end of the study. Echocardiograms were analyzed at the start and the end of the study. The study ended in February, 2010.
The KiSel-10 study had a gold standard design: it was a randomized, double-blind, placebo-controlled clinical trial. The results of the KiSel-10 study were reported in several medical journal articles in the period 2013 – 2015. Long-term supplementation of elderly citizens with SelenoPrecise® and Bio-Quinone Q10 resulted in significantly lower mortality rates, significantly lower cardiac natriuretic peptide levels, significantly better cardiac scores on echocardiograms, and significantly lower numbers of hospitalizations in the active treatment group as compared with the placebo group. More about the results of the KiSel-10 study later in this history.
2003: Rosenfeldt: systematic review of Coenzyme Q10 studies
At the same time that Dr. Mortensen was writing the biochemical rationale for the Q-Symbio study, Dr. Franklin L. Rosenfeldt of the Cardiac Surgical Research Unit, Alfred Hospital and Baker Institute, Melbourne, Victoria, Australia, was doing a systematic review of the effect of Coenzyme Q10 on cardiovascular disease, hypertension, and exercise performance [78].
- Coenzyme Q10 in Physical Exercise. Dr. Rosenfeldt identified 11 studies; six showed some modest improvement in exercise capacity with Coenzyme Q10 supplementation while five showed no effect.
- Coenzyme Q10 in Hypertension. Dr. Rosenfeldt identified eight published trials of Coenzyme Q10 in hypertension. In the eight studies, the mean reduction in systolic blood pressure was 16 mm Hg. The mean reduction in diastolic blood pressure was 10 mm Hg. Coenzyme Q10 had a role as an adjunctive treatment to conventional treatments in hypertension.
- Coenzyme Q10 in Heart Failure. Dr. Rosenfeldt did a meta-analysis of nine randomized trials. In those nine trials, there were non-significant trends towards increased ejection fraction and reduced mortality.

Dr. Franklin L. Rosenfeldt, Baker Heart Research Institute, Alfred Hospital, Monash University, Australia, has been accumulating laboratory and clinical evidence of the efficacy of Coenzyme Q10 treatment of various cardiovascular disorders for many years. Dr. Rosenfeldt was one of primary researchers in the Q-Symbio study.
2003: Rosenfeldt: Coenzyme Q10 and class II and III heart failure
Dr. Rosenfeldt and his colleagues reported on their own three-month randomized, double-blind, placebo-controlled pilot study of Coenzyme Q10 therapy in 35 patients with class II and class III heart failure. [43]. The intervention with Coenzyme Q10 yielded a threefold increase in the blood Coenzyme Q10 levels in the treated group; there was no increase in the placebo group. The patients treated with Coenzyme Q10 showed a statistically significant improvement of one-half NYHA functional class, compared with patients in the placebo group. They also showed significant improvement in their Specific Activities Scale class and in their C-min walk-test distances. The researchers noted a positive correlation between increases in serum Coenzyme Q10 concentrations and increases in exercise time [43].
2003: Zita, Mortensen, and Moesgaard: raising serum CoQ10 levels
Dr. Mortensen knew that he would need to raise patients’ serum Coenzyme Q10 concentrations to the level of about 2.5 mg/L in order to achieve a clinical effect [94]. That is the level needed in serum to have a sufficiently high concentration for the Coenzyme Q10 to leave the blood and enter the tissue.
Together with Dr. Zita of the Medical Faculty Hospital in Prague, Czech Republic, and Sven Moesgaard of Pharma Nord, Dr. Mortensen arranged to test the effect of supplementation with 30 and 100 milligrams of Coenzyme Q10 (Pharma Nord’s Bio Quinone Q10) in healthy male volunteers.
At baseline, the median serum CoQ10 concentration in the volunteers was 1.26 mg/L of serum, and concentration levels ranged from 0.82 (10th percentile) to 1.83 (90th percentile). After two months of supplementation in a randomized, double-blind, placebo-controlled study, the patients’ median increases in serum CoQ10 concentration were 0.55 mg/L for the 30-milligram dosage and 1.36 mg/L for the 100-milligram dosage.
The increases in the two Coenzyme Q10 treatment groups were significantly different from the slight decrease of 0.23 mg/L in the placebo group. Moreover, the supplementation-caused changes in serum Coenzyme Q10 concentrations were found to be independent of differences in baseline serum levels, age, or body weight.
In sum, supplementation with 30 milligrams per day brought the median serum Coenzyme Q10 level up to 1.81 mg/L, and 100 milligrams per day brought the median serum CoQ10 level up to 2.62 mg/L. Clearly, a daily dosage of 100 milligrams or more per day was indicated for future clinical trials.
2004: Balercia and Littarru: Increasing sperm motility
A team of Italian Coenzyme Q10 researchers enrolled 22 patients diagnosed with reduced sperm motility (mean age 31 years) in a 6-month-long open, uncontrolled pilot study [9]. They gave the patients Bio-Quinone Q10 200 mg/day (100 milligrams twice daily for 6 months) and recorded significantly increased Coenzyme Q10 levels in the patients’ plasma and in sperm cells. They documented a significant increase in sperm cell motility as well. They proposed that supplementation with Q10 be considered as a treatment option in cases of asthenozoospermia (reduced sperm motility).
2004: Berman: Coenzyme Q10 for heart transplant patients
Dr. Berman and colleagues at the Rabin Medical Center in Petah Tikva, Israel, enrolled 32 end-stage heart failure patients who were waiting for heart transplants in a randomized, double-blind, placebo-controlled study [10]. The patients received 60 milligrams of Coenzyme Q10 or placebo per day in addition to their regular medications. 27 patients completed the three-month program, and the patients in the Coenzyme Q10 treatment group showed significant improvements in the six-minute walk test as well as significant decreases in difficulty of breathing, in NYHA class, in the need to urinate at night, and in fatigue. Supplementation with Coenzyme Q10 improved functional status, symptoms, and quality of life in end-stage heart failure patients.
2005: Langsjoen: Use of Coenzyme Q10 and discontinuation of statin medications in cardiology clinic patients
The American cardiologist Dr. Peter H. Langsjoen of Tyler, Texas, evaluated 50 consecutive new cardiology clinic patients who had already been on statin drug therapy for an average of 28 months for possible adverse statin effects (muscle pain, fatigue, difficulty breathing, memory loss, and peripheral neuropathy)[51].
Dr. Langsjoen then discontinued the 50 patients’ statin therapy because of the adverse side effects of the statin medications, and he started them on supplemental Coenzyme Q10 at an average of 240 mg/day. He followed the patients for an average of 22 months with 84% of the patients followed for more than 12 months.
In the follow-up period, Dr. Langsjoen saw a drop in the prevalence of the symptoms that the patients reported on their initial visits. Fatigue decreased from 84% to 16%, muscle pain decreased from 64% to 6%, difficulty breathing decreased from 58% to 12%, memory loss decreased from 8% to 4%, and peripheral neuropathy decreased from 10% to 2%.
Heart function in the patients for whom the statin drug therapy had been discontinued either improved or remained stable in the majority of patients. There were no adverse consequences from the discontinuation of the statin drug therapy.
2005: Singh, Moesgaard, Littarru: Raising serum CoQ10 levels
Still looking to improve the uptake of Coenzyme Q10, Dr. Singh of the Halberg Hospital and Research Institute in Moradabad, India, conducted a randomized, double-blind, placebo-controlled clinical trial for 20 days [86]. The researchers enrolled 60 healthy men, aged 18-55 years, and tested various dosages and dose strategies using Pharma Nord’s Myoqinon 100 mg (same raw material and same formulation as in Bio-Quinone Q10) capsule and, for comparison purposes, crystalline 100 mg Q10 powder capsules or placebo capsules.
The Singh team of researchers found the following results:
- The patient compliance (checked by capsule counting) was above 90%
- The side effects of taking Q10 supplements were negligible
- The Q10 dissolved in oil (Myoqinon) was more effective than the same amount of crystal powder Q10 in raising serum Q10 levels
- A divided dose strategy of Myoqinon 100 milligrams twice a day (with breakfast and dinner) improved absorption by nearly 45% compared to a single dose of 200 milligrams of Myoqinon once a day (with dinner)
- Supplementation with 200 milligrams of Myoqinon Q10 for 20 days resulted in significantly reduced levels of malondialdehyde, a biological marker for oxidative stress
2005: Safety of Coenzyme Q10
Singh, Moesgaard, Littarru, et al summarized the studies of relatively high daily dosages of Coenzyme Q10 [86].
- Langsjoen (1994): doses ranging from 75 to 600 mg/day (mean 242 mg/day) with 424 cardiovascular disease patients with no apparent side effects except for one case of nausea [52]
- The Huntington’s Disease Study group (2001): doses ranging from 600 mg/day to 1200 mg/day for up to 30 months with no adverse effects [32]
- Shults et al (2002): doses ranging from 400 mg/day to 800 mg/day with no adverse effects reported and doses up to 1,200 mg/day for as many as 16 months with no significant side effects [82]
- Shults and Beal (2004): daily dosages of 1,200, 1,800, 2,400, and 3,000 mg/day together with a stable dosage of vitamin E (alpha-tocopherol) 1200 IU/day in 17 patients with Parkinson’s disease in an open-label study with the only side effects seemingly unrelated to the Coenzyme Q10 administration. The patients’ plasma Coenzyme Q10 levels reached a plateau at the 2400 mg/day dosage level [84]
A year later, Ikematsu (2006) reported doses up to 900 mg/day for four weeks safe and well tolerated [33].
2006: Observed daily safe upper limit for oral CoQ10
The Huntington’s Disease Study Group proposed that dosages of 2,400 mg/day may strike the best balance between tolerability and blood level achieved 29]. Somewhat later (2006), Hathcock and Shao proposed that 1,200 mg/day should be the observed safe upper limit for Coenzyme Q10 [32].
A safety assessment done by Kaneka researchers Hidaka and Hosoe (2008) established 12 milligrams of oral Coenzyme Q10 per kilogram of body weight per day as the acceptable daily intake. The researchers settled on 1,200 mg per day as a safe upper limit of [30]. Thus, a man weighing 165 pounds (75 kg) could, conceivably, take 900 mg (calculated as 75 kg times 12 mg) of Coenzyme Q10 daily.
Hidaka and Hosoe examined evidence from pharmacokinetic studies that show that orally ingested CoQ10 does not influence the biosynthesis of Coenzyme Q10 and does not accumulate in plasma or tissues after the cessation of supplementation [30]. Hidaka and Hosoe concluded that Coenzyme Q10 is highly safe for use as a dietary supplement based on data from preclinical and clinical studies.
2006: Sander: Second meta-analysis of CoQ10 and heart failure
Dr. Soja and Dr. Mortensen published the first meta-analysis of studies of Coenzyme Q10 supplementation and heart failure in 1997 [81]. Dr. Stephen Sander of the University of Connecticut’s School of Pharmacy in Storrs, Connecticut, and his colleagues did a second meta-analysis of 11 clinical trials to evaluate the impact of CoQ10 therapy on ejection fraction and cardiac output [79].
The pooled data showed a statistically significant 3.7% net improvement in ejection fraction with an even better improvement noted in patients who were not receiving angiotensin-converting enzyme inhibitors. The data also showed a significantly increased cardiac output. The researchers concluded that supplementation with Coenzyme Q10 enhances systolic function in chronic heart failure.
2006-2007: Coenzyme Q10 in its reduced form, ubiquinol
In 2006, the Kaneka company introduced Coenzyme Q10 in its reduced form, ubiquinol, as a commercial product. This move was puzzling for several reasons:
- The lack of any documented effect for ubiquinol – all of the studies showing beneficial health effects had been done with the oxidized form of Coenzyme Q10, the ubiquinone form
- The known instability of the ubiquinol molecules (a common characteristic of antioxidants)
- The absence of any physical explanation for any supposed superiority of the ubiquinol product
- The greater cost of producing the ubiquinol raw material and finished products
- The knowledge that the ubiquinol in supplements is converted to ubiquinone in the stomach and the small intestine prior to absorption
- The knowledge that the ubiquinone that enters the absorption cells in the small intestine is converted to ubiquinol as it passes into the lymph – it is not necessary to ingest ubiquinol in order to get ubiquinol in the blood
2007: Dr. Judy on Coenzyme Q10 facts and fabrications
In 2007, Dr. William Judy of the SIBR Research Institute, himself the holder of a Ph.D. in human physiology and bio-physics, addressed many of the marketing claims for the newly introduced ubiquinol product [41]. Dr. Judy was concerned that some of the marketing claims for ubiquinol supplements were factual but not functionally important and that other marketing claims for ubiquinol supplements were more fabrication than fact.
Dr. Judy made the following points about Coenzyme Q10 supplements [41]:
- The body cannot absorb Coenzyme Q10 crystals; only single Coenzyme Q10 molecules can be absorbed.
- The important thing is to produce Coenzyme Q10 supplements that have the raw material dissolved in vegetable oils in such a way that the Coenzyme Q10 does not re-form into crystals at body temperature or room temperature.
- The melting point of Coenzyme Q10 is approximately 10 degrees centigrade higher than body temperature, and a body temperature of 47 degrees centigrade is incompatible with life.
- Coenzyme Q10 cannot be made into water-soluble molecules and continue to be Coenzyme Q10. The two hydrogen ions on the polar head of the ubiquinol molecule may make it slightly more water-soluble than the ubiquinone molecule, but the ubiquinol molecule continues to be far more lipid-soluble than water-soluble because of the larger non-polar tail of the molecule.
- Ubiquinol (reduced Coenzyme Q10) is highly unstable and is concerted to ubiquinone in the stomach before it ever reaches the absorption cells in the small intestine. In fact, the ubiquinol in some products is converted to ubiquinone in the softgel capsule.
- Coenzyme Q10, whether ingested in the form of ubiquinone or ubiquinol, leaves the stomach in the form of ubiquinone. In the absorption cells in the small intestine, and in the distal lymph, almost all of the ubiquinone being absorbed is converted to ubiquinol.
- The absorbed Coenzyme Q10 is transported from the absorption cells to the lymph and from the lymph to the venous blood.
- Coenzyme Q10 concentrations peak in the lymph 2 -3 hours after ingestion and in the blood 5 – 8 hours after ingestion.
- 90 – 95% of the Coenzyme Q10 in the circulating blood is in the form of ubiquinol, regardless of the form in which it was ingested.
- The energy producing form of Coenzyme Q10 is the ubiquinone form. Because the body does not need to produce energy in the lymph and in the blood, it is not surprising that the Coenzyme Q10 is in the form of ubiquinol, which is the form of Coenzyme Q10 that provides antioxidant protection against the peroxidation of the lipids being transported in the blood.
- Coenzyme Q10 accumulates in the blood and becomes bioavailable to the cells. It is stored in the cell membranes and in the membranes of cellular organelles.
- There is no solid evidence that the absorption or resultant bio-availability of the best-formulated Coenzyme Q10 in the ubiquinol form is greater than the absorption and bioavailability of the best-formulated ubiquinone forms. In fact, the best-formulated ubiquinone supplements may yield a somewhat better absorption and bioavailability.
- Many of the claims for the superiority of the ubiquinol form are the result of comparisons of differing formulations of the two products. The question is whether the absorption of CoQ10 should be measured from CoQ10 concentrations in the blood or from CoQ10 concentrations in the distal lymph, which is adjacent to the absorption cells
- In the inner membranes of the mitochondria, the ubiquinol is rapidly converted to ubiquinone. In the mitochondria, there is a great demand for the ubiquinone form of Coenzyme Q10 to serve as a carrier of electrons and protons in the process of producing ATP molecules. The conversion of Coenzyme Q10 from ubiquinol to ubiquinone and back again creates the Q-cycle described by Dr. Peter Mitchell in the work for which he received the Nobel Prize in chemistry in 1978 (see above).
- The body cannot store ATP, so it must be continuously produced. Ubiquinone, the oxidized form of Coenzyme Q10, is a major co-factor in the cellular process of energy synthesis (ATP). Ubiquinol cannot replace ubiquinone in this process because the ubiquinol is not an electron acceptor.
- What ubiquinol can do is regenerate ubiquinone and Vitamin C and Vitamin E in the body. Ubiquinol is also a powerful antioxidant that protects the body against toxic oxidative reactions.
2007: Dr. Judy’s conclusion
Dr. Judy stated, with respect to the new ubiquinol products, that consumers of Coenzyme Q10 supplements should take certain factors into consideration. Among these factors, he listed the lack of a documented superior absorption, the known instability in the stomach, the absence of clinical trials documenting health effects, and the higher cost of the ubiquinol products.
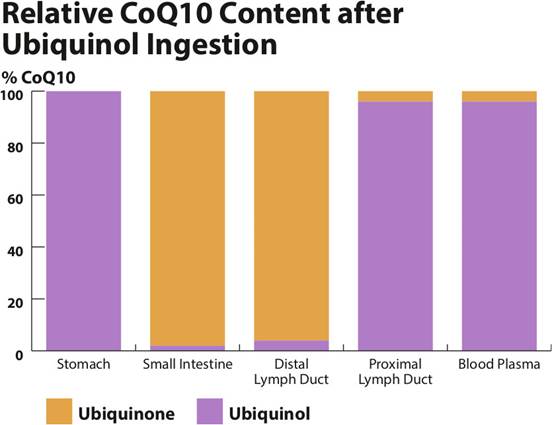
|
Location |
CoQ10 as ubiquinol |
CoQ10 as ubiquinone |
|
Stomach |
100% |
0% |
|
Small intestine |
2% |
98% |
|
Distal lymph duct |
4% |
96% |
|
Proximal lymph duct |
96% |
4% |
|
Blood plasma |
96% |
4% |
The above-pictured graph shows Dr. Judy's and Dr. Stogsdill’s measurements of the percentages of ubiquinol and ubiquinone during the transfer of ingested ubiquinol in large animals from the stomach to the blood. Following the ingestion of ubiquinol, the CoQ10 begins to be converted to ubiquinone in the stomach because of the high hydrogen ion concentration. In the small intestine, the CoQ10 is almost entirely in the form of ubiquinone. In the distal lymph duct, the CoQ10 is, initially, in the ubiquinone form. The absorbed ubiquinone then begins to be converted to ubiquinol. By the time that the CoQ10 has passed from the lymph to blood, the total ubiquinol percentage is 96%, and the ubiquinone percentage is 4%. These data show the redox conversion of ubiquinol to ubiquinone in the stomach and small intestine and the conversion from ubiquinone to ubiquinol in the lymph ducts on the way to the systemic circulation. Dr. Judy presented these data to the membership of the International Coenzyme Q10 Association at the association’s 8th international conference held in Bologna, Italy, in October of 2015.
2008: Molyneux: Low plasma Coenzyme Q10 an independent predictor
Dr. Sarah L. Molyneux of the Canterbury Health Laboratories in Christchurch, New Zealand, tested the hypothesis that low plasma Coenzyme Q10 concentrations predict increased incidence of all-cause mortality in chronic heart failure patients. She and her colleagues examined the plasma samples and case histories of 236 heart failure patients admitted to hospital [63].
They found that low plasma Coenzyme Q10 levels predicted mortality independent of other factors such as age, gender, previous heart attack, cardiac natriuretic peptide levels, and renal disease. According to Dr. Molyneux’ calculations, the optimal value for the prediction of mortality was ≤ 0.73 micromol/L, which is equivalent to ≤ 0.63 mg/L.
Dr. Svend Aage Mortensen has pointed out that, in the Molyneux study, the association between the low CoQ10 concentrations and mortality in heart failure patients is even stronger than the association between N-terminal pro–B-type natriuretic peptide levels and mortality in heart failure patients [69]. NT-proBNP is a marker for atrial and ventricular distension caused by increased pressure inside the heart.
Given that such a relationship exists between plasma Coenzyme Q10 levels and chronic heart failure outcomes, it is important to monitor heart failure patient plasma levels and to use oral supplementation to increase plasma concentrations to levels that enable the transfer of the Coenzyme Q10 from the blood to the tissues.
Dr. William Judy of SIBR Research Institute says that a plasma concentration of 2.5 micrograms per milliliter is typically needed for the Coenzyme Q10 to go into the tissues.
Dr. Steven Sinatra has extrapolated from the research results of Dr. Peter Langsjoen that a blood Coenzyme Q10 level of 2.5 - 2.9 micrograms per milliliter is needed for optimal improvement of class III and IV heart failure patients [84].
2008: Adarsh: Coenzyme Q10 and hypertrophic cardiomyopathy
Hypertrophic cardiomyopathy is a disease in which a portion of the heart muscle becomes thicker, and the thickening of the muscle makes the pumping of blood more difficult. Dr. Kumar Adarsh of the Government Medical College in Amritsar, India, recruited 46 patients with hypertrophic cardiomyopathy and added 200 milligrams of Coenzyme Q10 to their conventional medications [2]. He then matched the 46 patients, by age and sex and disease condition, with 41 similar patients who received only the conventional medications. All of the patients were classified as NYHA class II or higher. Dr. Adarsh then observed the patients for an average of 14 months (range: from 9 months to 27 months).
In the treatment group that received the Coenzyme Q10 supplementation, Dr. Adarsh observed significant improvement in quality of life on the six-minute walk test, in NYHA functional class, in diastolic function, and in mitral regurgitation. Diastolic pressure is the blood pressure measured when the heart is resting (as opposed to systolic pressure when the heart is contracting). Mitral regurgitation refers to a heart valve condition in which the blood leaks backwards through the mitral valve when the heart contracts.
Dr. Adarsh and his colleagues concluded that supplementation with Coenzyme Q10 is a safe, effective, and beneficial adjuvant therapy for diastolic heart failure in patients of hypertrophic cardiomyopathy.
2009: Kocharian: Coenzyme Q10 for children with heart disease
Dr. Kocharian and colleagues found that adding Coenzyme Q10 supplementation to conventional therapy was beneficial for children under the age of 18 who had been diagnosed with heart failure caused by idiopathic dilated cardiomyopathy (decreased ability of the heart to pump blood because of an enlarged and weakened left ventricle)[45].
Dr. Kocharian and the team of researchers did a randomized, double-blind, placebo-controlled trial in which 17 children received Coenzyme Q10 and 21 children received placebo. The children who received the Coenzyme Q10 started out with a dosage of a dose of 2 milligrams per kilogram of body weight per day in 2 or 3 divided daily doses for six months. The dosage was then gradually increased up to a maximum dosage of 10 milligrams per kilogram per day as long as the children tolerated the increases. The researchers reported that the Coenzyme Q10 was well tolerated, and there were no adverse effects
After six months of supplementation, the children who had received the Coenzyme Q10 supplements showed significant improvement in their index scores for cardiac failure and in their diastolic function.
2010: McMurray: Coenzyme Q10 in the CORONA study
In the Controlled Rosuvastatin Multinational Study in Heart Failure (CORONA) study, Dr. McMurray and colleagues at the Glasgow Cardiovascular Research Centre, University of Glasgow, in the United Kingdom measured the serum Coenzyme Q10 concentrations in 1,191 patients with ischemic systolic heart failure [60]. They reported the following results:
- Patients who had lower serum COQ10 levels were older patients who had more advanced heart failure.
- There was a significantly higher mortality rate in the lowest serum CoQ10 tertile compared to the highest serum CoQ10 tertile.
- The use of the statin medication Rosuvastatin did reduce serum CoQ10 levels.
- Patients in the lowest serum CoQ10 tertile had significantly lower left ventricular ejection fractions.
- Patients in the lowest serum CoQ10 tertile had higher natriuretic peptide levels. (Higher B-type natriuretic peptide levels are an indicator of volume expansion and pressure overload in the heart.)
Dr. Michael Felker of the Duke Heart Center in Durham, North Carolina, concluded from the results of the CORONA study that serum CoQ10 levels might be good indicators of the severity of heart failure even if serum CoQ10 levels are not necessarily useful for prognostic purposes in and of themselves [80].
Dr. Svend Aage Mortensen pointed out that 10 milligrams of Rosuvastatin treatment per day in the CORONA study had reduced the plasma Coenzyme Q10 concentration significantly by 39%. That reduction had lowered the median Coenzyme Q10 level below the baseline level of Coenzyme Q10 in the CORONA patients who were classified in lowest Coenzyme Q10 tertile -- 0.48 microg/mL – a level that seemed to indicate tissue depletion of Coenzyme Q10.
2010: Rosenfeldt: Coenzyme Q10 before and after surgery
Dr. Rosenfeldt and research colleagues in the Cardiac Surgical Research Unit at the Alfred Hospital in Melbourne, Australia, enrolled 117 heart surgery patients – scheduled for coronary artery bypass graft and/or valve surgery – in a randomized, double-blind, placebo-controlled trial [55]. For two months prior to surgery and for one month following surgery, the patients received either a combination of Coenzyme Q10, magnesium, lipoic acid, fatty acids, and selenium or corresponding placebos.
On average, the heart surgery patients got the combination therapy for 76 ± 7.5 days. The combination therapy containing Coenzyme Q10 and selenium significantly reduced plasma troponin levels 24 hours after the heart operations. Troponins are proteins released into the blood whenever the heart muscle has been damaged. The greater the damage, the greater the level of troponins in the blood.
The combination therapy containing Coenzyme Q10 and selenium also significantly shortened the mean length of postoperative hospital stay by 1.2 days, which resulted in reduced hospital costs for each heart operation.
The supplements used in the combination metabolic therapy were relatively inexpensive and were safe. There were no clinically significant side effects in the active treatment group. [55]
2012: Deichmann: Coenzyme Q10 and statins and exercise
In addition to causing reductions in the biosynthesis of Coenzyme Q10, statin medications have the potential to cause muscle toxicity. Dr. Richard Deichmann of the Ochsner Medical Center in New Orleans investigated the possible effect of statin medications on exercise performance in athletes aged 50 years or older [13].
He and his colleagues randomly assigned 20 older athletes who were taking statin medications to receive 200 milligrams of Coenzyme Q10 or corresponding placebo daily in a six-week double-blind, placebo-controlled cross-over trial. Supplementation with Coenzyme Q10 did not significantly improve the athletes’ anaerobic threshold but did significantly improve change time to anaerobic threshold and did significantly improve leg strength as measured by quadriceps muscle extension repetitions.
2012 – 2013: Lee: Coenzyme Q10 and statins
Statin medications reduce the biosynthesis of Coenzyme Q10. Coenzyme Q10 plays an important role in cellular bio-energetics and as an antioxidant. Dr. Lee and a team of researchers from the Chung Shan Medical University in Taichung, Taiwan, investigated whether daily supplementation with 300 milligrams of Coenzyme Q10 (taken as 150 milligrams twice a day) could enhance antioxidant enzyme activity and reduce markers of inflammation in coronary artery disease patients who were taking statin medications [54].
Supplementation significantly increased the plasma Coenzyme Q10 levels and the levels of the antioxidant enzymes superoxide dismutase, catalase, and glutathione peroxidase. Supplementation also significantly reduced the levels of the inflammatory markers C-reactive protein, tumor necrosis factor-α, and interleukin-6.
Dr. Lee and his colleagues reported on an earlier study in which patients with coronary artery disease – patients having at least 50% narrowing of one major coronary artery or receiving coronary angioplasty – received 150 milligrams of Coenzyme Q10 daily for 12 weeks [53].
The results of the study showed that Coenzyme Q10 supplementation significantly reduced the levels of malondialdehyde (a marker for oxidative stress) and significantly increased the levels of the antioxidant enzymes catalase and superoxide dismutase.
2013: Fedacko and Pella and Littarru: Coenzyme Q10 and selenium and patients on statin medications
Drs. Jan Fedacko and Daniel Pella of the Pavol Jozef Safarik University in Kosice, Slovakia, and Dr. Gian Paolo Littarru of the Polytechnic University of The Marche in Ancona, Italy, carried out a randomized, double-blind, placebo-controlled clinical trial with 60 statin medication patients who were suffering from muscle pain, muscle weakness, tiredness, or muscle cramps [20].
The researchers gave the statin patients either a combination of 200 milligrams/day Coenzyme Q10 capsules (Bio-Quinone Q10 100 milligrams twice daily) and 200 micrograms/day of organic selenium yeast tablets (SelenoPrecise®), or corresponding placebo capsules and tablets. The patients continued to take their regular prescribed medications.
The results showed that supplementation of statin-treated patients with Coenzyme Q10 and selenium diminished the symptoms of muscle pain and fatigue, which are associated with the taking of statin medications.
2013: Bogsrud: Statins, Coenzyme Q10 and selenium, and statin-induced muscle pain and fatigue
In a 12-week randomized, double-blind, placebo-controlled study, Dr. Bogsrud and a team of Norwegian researchers from Ålesund Hospital, Ålesund, Norway, found that the administration of the statin medication atorvastatin reduced significantly the patients’ blood Coenzyme Q10 levels in both groups [11]. Supplementation with 400 mg/day of Myoqinon then significantly increased CoQ10 levels in the active treatment group.
Atorvastatin did not decrease blood selenium levels in either group. Supplementation with SelenoPrecise® increased blood selenium levels in the active treatment group.
After 12 weeks of supplementation that was first begun after the patients had experienced the pain and fatigue associated with the taking of atorvastatin, the results from symptom questionnaire scores and muscle function tests did not show significant differences between the treatment group and the placebo group with respect. However, four patients in the placebo group did stop the statin treatment because of unbearable pain from the statin medication. No patients in the treatment group stopped the statin treatment. This difference was not statistically significant but might be clinically significant.
Moreover, atorvastatin’s effect in decreasing CoQ10 concentrations may have long-term implications for heart muscle function. Because the taking of statin medications so clearly decreases the patients’ biosynthesis of Coenzyme Q10, patients on statin medications need Coenzyme Q10 supplements to protect the heart muscle function.
2013: Alehagen and the KiSel-10 study in Sweden:
Coenzyme Q10 and selenium for elderly citizens
Starting in 2003 and ending in 2010, Dr. Urban Alehagen and colleagues at the University Hospital in Linköping, Sweden, enrolled 443 healthy elderly Swedish citizens aged 70 to 88 in a randomized, double-blind, placebo-controlled study of the effects of supplementation with Coenzyme Q10 (Bio-Quinone Q10 100 mg twice daily) and with organic selenium yeast (SelenoPrecise® 200 micrograms once daily). They called the study the KiSel-10 study [3].

Professor Urban Alehagen, Department of Cardiology, Linköping University Hospital, lead researcher on the KiSel-10 study and one of the primary researchers on the Q-Symbio study
In 2013, the researchers reported the statistically significant outcomes of the supplementation [3]:
- Reduction in cardiovascular deaths
- Reduction in levels of NT-proBNP (a biological marker for heart disease)
- Better cardiac function scores on echocardiograms
- Fewer hospitalizations
In subsequent journal articles, Dr. Alehagen and his colleagues reported that the combination treatment with selenium and Coenzyme Q10 is positively associated with improvement in bio-markers for inflammation [6] and for oxidative stress [7]. They also reported that the combination treatment had resulted in fewer days in hospital and in better quality of life for the participants in the study [35]. Moreover, the positive health effects of the treatment had persisted ten years after the start of the four-year treatment period [4].

Follow-up of the participants in the KiSel-10 study for a period of 10 years after the initiation of the four-year active treatment showed that the effect on cardiovascular mortality four years of supplementation with Bio-Quinone Q10 and SelenoPrecise® persisted well after the end of the supplementation.
In a 2015 follow-up article, Drs. Alehagen and Aaseth summarized what they had learned from the KiSel-10 study results and from their review of the relevant research literature [5]:
- There is an important interrelationship between Coenzyme Q10 and selenium. Selenium deficiencies can inhibit the cells from getting optimal concentrations of Coenzyme Q10, and adequate concentrations of Q10 must be available for the cells to benefit from optimal selenium function.
- The available literature shows unequivocally that statin treatment leads to decreases in serum Q10 levels and that statin treatment leads to the inhibition of the synthesis of some important selenoproteins.
- The risk of developing heart disease is positively associated with low serum Q10 levels and low serum selenium levels.
- The clinical results from the KiSel-10 intervention study using both selenium and coenzyme Q10 in an elderly population were statistically significant: a reduction in cardiovascular mortality, an improved cardiac function shown in echocardiograms, and slower increases in heart muscle wall tension shown in lower concentrations of the biomarker NT-proBNP.
- Coenzyme Q10 (in its reduced form) is an important antioxidant, and selenium is an essential component of some important antioxidant enzymes. Antioxidant protection is especially important in the prevention of heart disease because the mitochondrial DNA in the heart muscle cells is especially susceptible to damage caused by oxidative stress (oxidative stress = an imbalance between the body’s production of free radicals and the body’s ability to neutralize the free radicals).
- There is an interrelationship between selenium and Coenzyme Q10 that can be exploited for therapeutic advantage if both substances are used together. The combination of selenium and Coenzyme Q10 supplementation is appropriate as a preventive measure in middle-aged and elderly persons at risk for developing heart disease and as an adjunctive treatment of patients diagnosed with heart failure.
2013: Fotino: A third meta-analysis of Coenzyme Q10 adjuvant treatment of heart failure patients
Dr. Soja did the first meta-analysis of Coenzyme Q10 and heart failure studies in 1997 [87]. Dr. Sander did the second meta-analysis in 2006 [79]. In 2013, Dr. Dominica Fotino and researchers from Tulane University did a third systematic review of randomized controlled trials of CoQ10 supplementation of heart failure patients [26]. Dr. Fotino focused on studies that reported the ejection fraction (EF) or NYHA functional class of patients as a primary outcome. The researchers pooled data from 13 clinical trials enrolling 935 heart failure patients.
The pooled data from the 13 studies showed an improvement in left ventricular ejection fraction of 3.67% in the CoQ10 treatment group as contrasted with the placebo group [26].
2013: Madmani: Cochrane review
Dr. Mohammed Eid Madmani of Case Western Reserve University School of Medicine, Cleveland, Ohio, reviewed the safety and efficacy of Coenzyme Q10 adjuvant treatment in heart failure as compared to placebo in seven randomized controlled trials with 914 participants [61]. Five of the seven studies reviewed by Dr. Madmani were among the 13 studies reviewed by Dr. Fotino [26].
The pooled data from the seven studies demonstrated that supplementation with Coenzyme Q10 increased blood levels significantly without causing any adverse alterations of the patients’ hemodynamic status and without raising any concerns about other safety issues. However, the results from the seven studies did not show significant improvement in left ventricular ejection fraction or exercise capacity.
Both the Fotino meta-analysis and the Madmani review included the results of the Khatta study published in 2000. The Khatta study results remain a puzzle [44]. The supplementation with 200 milligrams of Coenzyme Q10 daily for six months did increase the mean serum CoQ10 levels from 0.95 +/- 0.62 mg/L to 2.2 +/- 1.2 mg/L. However, the CoQ10 supplementation did not significantly change the patients’ ejection fraction, peak oxygen consumption, and exercise duration.
One probable explanation for the unexpected results is non-compliance by many of the patients in the active treatment group.
In Figure 2 of the Khatta study report, it can be seen that that, of the 22 patients who were assigned to the Coenzyme Q10 treatment group, one patient showed a decline in Coenzyme Q10 concentration, nine did not increase their CoQ10 levels beyond 1.0 mg/L, five more did not increase their CoQ10 levels above 1.5 mg/L, and two patients pushed their CoQ10 levels close to 2.0 mg/L.
Only five patients achieved CoQ10 levels above 2 mg/L, and, of those five, only three achieved CoQ10 levels above 2.5 mg/L, the level at which the Coenzyme Q10 is most likely to pass from the blood to the tissue.
As was indicated above, the American cardiologist Dr. Steven T. Sinatra has estimated from the results of available studies that the therapeutic blood levels of Coenzyme Q10 need to be at least 2.5 mg/L to produce a significant response [84]. At the end of the Khatta study, 19 of the 22 patients (86%) in the CoQ10 treatment group had blood levels lower than 2.5 mg/L, the minimal therapeutically effective level. The most likely explanation is that there was considerable non-compliance in the Khatta study [44].
2013: Fotino and Madmani reviews published prior to Q-Symbio study
Both of the 2013 reviews, by Dr. Fotino [26] and by Dr. Madmani [61], were completed before the publication of the results of the Q-Symbio study of the effect of Coenzyme Q10 on morbidity and mortality in chronic heart failure [70]. Both of the 2013 reviews of the literature have to be viewed as having come too soon. Inclusion of the Q-Symbio study – with its 420 heart failure patients the biggest randomized controlled trial of Coenzyme Q10 and heart failure since the 1993 Morisco study – would have made for different outcomes.
2014: Mortensen: publication of the results of the Q-Symbio trial in the Journal of the American College of Cardiology
Going into the Q-Symbio study of the morbidity and mortality of supplementation with Coenzyme Q10 in chronic heart disease [70], Dr. Mortensen already had biochemical data and explanations to draw on.
I. Coenzyme Q10 plays important roles in the body:
- It is necessary for the production ATP in the cells.
- It is an antioxidant that quenches harmful free radicals.
- It promotes cell membrane stability.
- It plays a role regulating certain genes.
II. Low blood and tissue CoenzymeQ10 concentrations have been shown to be associated with the development and progression of chronic heart failure.
III. Several factors explain how the heart failure patients came to have low blood and tissue Coenzyme Q10 concentrations.
- Biosynthesis of Coenzyme Q10 decreases in step with increasing age, beginning in the early 20s.
- Patients may be getting less Coenzyme Q10 in their food.
- There may be an increased demand for Coenzyme Q10 caused by the compensatory neuro-hormonal responses to lower cardiac output.
- Coenzyme Q10 may be used to excess as an antioxidant in heart tissues subjected to oxidative stress.
- Statin therapy may be inhibiting the body’s synthesis of Coenzyme Q10.
IV. Conventional pharmaceutical methods of reducing cardiac dysfunction are all intended to block cellular processes. Adjuvant therapy with Coenzyme Q10, on the other hand, enhances the cellular processes of energy production and antioxidation.
V. Randomized controlled trials of Coenzyme Q10 adjuvant therapy in chronic heart failure, enrolling a total 988 patients, had shown patient improvement in such clinical parameters such as New York Heart Association (NYHA) functional classification, exercise capacity, and quality of life. Meta-analyses of randomized controlled trials of Coenzyme Q10 supplementation of heart failure patients showed a positive effect of the adjuvant treatment on left ventricular ejection fraction.
VI. Plasma samples from 236 heart failure showed an independent association between low plasma Coenzyme Q10 levels and increased risk of mortality in chronic heart failure. The strength of the association between plasma Coenzyme Q10 levels and heart failure mortality was greater than the strength of the association between plasma NT-proBNP levels and heart failure mortality (concentrations of NT-proBNP in the blood are a biological marker for the severity of heart failure).
2014: Mortensen: statistically significant results from the Q-Symbio study
Q-Symbio was a randomized double-blind multicenter clinical trial of Coenzyme Q10 as an adjunctive treatment of chronic heart failure with a focus on changes in SYMptoms, BIomarker status (BNP) and long-term Outcomes such as hospitalizations and quality of life [70].
The researchers enrolled 420 heart failure patients in heart centers in Europe, Asia, and Australia and randomly assigned the patients to receive 300 milligrams of Myoqinon (same raw material and formulation as Bio-Quinone Q10) as 100 milligrams three times daily for two years or corresponding placebo capsules.
When the seal had been broken on the coded patient lists, the adjunctive treatment with Coenzyme Q10 was shown to have achieved the following statistically (and clinically) significant outcomes:
- A reduction in the number of major adverse cardiovascular events
- A reduction in the number of cardiovascular deaths
- A reduction in the all-cause mortality among the heart failure patients
- Fewer admissions to hospital
The published results of the Q-Symbio clinical trial (2014) replicate the earlier results in the Morisco trial (1993) and the Munkholm trial (1999).
2014: Golomb: Coenzyme Q10 supplementation for veterans diagnosed with Gulf War Illness
Dr. Beatrice Golomb of the University of California, San Diego in La Jolla, California, carried out a 3.5 month-long randomized, double-blind, placebo-controlled clinical trial of 46 Gulf War veterans who met both the Kansas and the Centers for Disease Control criteria for Gulf War illness [27]. She gave the veterans in the active treatment group 100 milligrams of Bio-Quinone Q10 per day.
Supplementation with 100 milligrams of Coenzyme Q10 per day was associated with improvement in physical function and symptoms in veterans with Gulf War illness. Among males (85% of enrollees), there were statistically significant benefits from 100 mg/day of Bio-Quinone Q10 on General Self-Rated Health and on physical function scores.
In 19 of 20 symptoms (sleep problems being the exception), Coenzyme Q10 supplementation was associated with signs of improvement, with several of the symptoms showing statistically significant improvement.
2014: Brauner: Coenzyme Q10 and patients with diabetes
Dr. Hanna Brauner and colleagues at the Karolinska Institute in Stockholm, Sweden, tested whether Bio-Quinone Q10 100 milligrams twice daily for 12 weeks is a beneficial supplement to patients with diabetes [12]. The researchers’ data suggest that Coenzyme Q10 supplementation can boost the immune system in Type 1 diabetics, can reduce diabetes-associated inflammatory processes, and may help prevent late complications.
The researchers observed signs of reduced inflammation, increased cytokine production capacity, improved NK cell activity, and reduced hBD2 expression in Type 1 diabetics receiving daily Q10 supplements. (hBD2 expression is indicative of pro-inflammatory activity)
2014: Del Pozo-Cruz and Navas: Coenzyme Q10 and exercise capacity in elderly people
Drs. Del Pozo-Cruz and Navas and researchers in Auckland, New Zealand and Sevilla, Spain, reported on the results of a study of how physical activity in the elderly affects endogenous Coenzyme Q10 levels in blood plasma [14]. Their findings show that elderly people with greater exercise capacity also have lower levels of cholesterol and lipid peroxidation and higher levels of Coenzyme Q10 in plasma.
In elderly people who are more active, the Coenzyme Q10/cholesterol and Coenzyme Q10/LDL ratios are higher. Obese elderly people tend to have lower plasma Coenzyme Q10 levels and higher lipid peroxidation levels, as measured by malondialdehyde levels in plasma. The data show that physical activity can increase the levels of Coenzyme Q10 and lower the levels of lipid peroxidation in plasma in elderly people.
2014: Pourmoghaddas: a combination of atorvastatin and Coenzyme Q10
Dr. Masoud Pourmoghaddas of the Isfahan Cardiovascular Research Institute, Isfahan University of Medical Sciences, in Isfahan, Iran, and colleagues conducted a randomized, double-blind, placebo-controlled trial in which they enrolled 62 eligible patients [77]. The patients in the intervention group received 10 milligrams atorvastatin daily plus 100 milligrams of a Coenzyme Q10 supplement twice daily. The placebo group patients received 10 milligrams atorvastatin daily and a placebo. The trial lasted 4 months.
There were significant improvements in ejection fraction and NYHA functional class in the intervention group as compared with the atorvastatin only group. Dr. Pourmoghaddas and his colleagues concluded that the combination of atorvastatin and Coenzyme Q10 has a better effect on ejection fraction and NYHA classification than atorvastatin alone.
2015: Okuyama and Langsjoen: Continuing concern about the effect of statin medications on Coenzyme Q10 levels and atherosclerosis
Dr. Harumi Okuyama of the Kinjo Gakuin University College of Pharmacy in Nagoya, Japan, and Dr. Peter H. Langsjoen collaborated on an article spelling out the possible pharmacological mechanisms by which the adverse effects of cholesterol-lowering statin medications might be causing more and worse cases of chronic heart failure and atherosclerosis [75].
- Statin medications inhibit the body’s endogenous production of Coenzyme Q10, which is essential for the cellular production of ATP.
- Statin medications inhibit the body’s production of vitamin K2, which is a cofactor for matrix Gla-protein activation, which protects the arteries from calcification.
- Statin medications inhibit the biosynthesis of selenium containing proteins, e.g. the glutathione peroxidase antioxidant enzyme that helps suppress peroxidative stress.
Drs. Okuyama and Langsjoen are concerned that the impairment of Coenzyme Q10 and selenoprotein biosynthesis by statin medications may be a factor in the development of congestive heart failure and atherosclerosis. Consequently, they propose that current statin treatment guidelines be critically reevaluated.
2015: DiNicolantonio: Review of Coenzyme Q10 and heart failure literature
Dr. James J. DiNicolantonio, a cardiovascular research scientist at Saint Luke's Mid-America Heart Institute in Kansas City, Missouri, did a systematic literature review [16]. He found that numerous randomized controlled trials of supplemental CoQ10 in heart failure showed improvements in functional parameters such as ejection fraction, stroke volume, and cardiac output without side effects. At least three meta-analyses confirmed those findings. Then the multi-center randomized controlled Q-Symbio trial showed significantly reduced major adverse cardiovascular events after 106 weeks of supplementation with 300 milligrams of Myoqinon (same raw material and formulation as Bio-Quinone Q10) taken three times daily in 100-milligram capsules.
Dr. DiNicolantonio’s summation of the Coenzyme Q10 and heart failure literature: given the excellent tolerance and the affordability and the documented health benefits of the natural physiological compound, Coenzyme Q10, adjuvant treatment of heart failure patients with supplemental Coenzyme Q10 has become an attractive option for cardiologists in the management of heart failure.
2015: Mortensen: Call for new heart failure treatment guidelines
In his letter to the journal JACC Heart Failure, Dr. Mortensen began by stating that heart failure is a disabling disease that robs its victims of energy and quality of life [71]. Heart failure is a disease with a poor prognosis in spite of the advances in medical drug and medical device treatment options.
Dr. Mortensen then reminded his readers that supplementation with Coenzyme Q10 in addition to the conventional heart failure therapy has been shown to improve symptoms, improve survival, and reduce hospitalization rates when compared with placebo supplementation in randomized controlled clinical trials.
He pointed out, furthermore, that there have been no reports of serious side effects in any of the more than 200 randomized controlled trials of Coenzyme Q10 supplementation that have been indexed in the Medline database.
To calls for additional clinical trials of the efficacy of Coenzyme Q10 as an adjuvant treatment in heart failure, Dr. Mortensen responded by saying that it is very difficult to raise funding for a large-scale trial of a non-patentable substance like Coenzyme Q10.
Moreover, and more importantly, he raised the question of whether it would be ethical, given the survival data from the Q-Symbio trial, for cardiologists to wait for the results of another trial before recommending Coenzyme Q10 supplementation to their heart failure patients. The further question is whether it is defensible to give half of the patients in a future clinical trial placebo capsules when it is known that Coenzyme Q10 supplementation benefits heart failure patients.
Already in 2005, the heart failure treatment guidelines promulgated by the American College of Cardiology and the American Heart Association acknowledged that supplementation with Coenzyme Q10 might have a positive effect, but the 2013 guidelines stopped short of recommending Coenzyme Q10 supplementation until more data were available [1].
Now, the positive data from the Q-Symbio trial and from the meta-analyses done by Dr. Soja [87], Dr. Sander [79], and Dr. Fotino [26] provide the necessary documentation for recommending Coenzyme Q10 as an adjunctive treatment in heart failure.
One of the best aspects of Coenzyme Q10 supplementation is that it works with the cells to restore a deficiency state – low blood and tissue Coenzyme Q10 levels are associated with heart failure – and it improves the bio-energetic processes and antioxidant processes in the heart muscle tissue.
2016: Felker: Duke University review of the status of adjuvant Coenzyme Q10 treatment in heart failure
Dr. Michael Felker and his colleagues at the Division of Cardiology, Duke University School of Medicine, Duke Heart Center in Durham, North Carolina, published a state-of-the-art review of the Coenzyme Q10 and heart failure literature [80]. They summarized the literature about the mechanisms, the clinical data, and the safety profile of Coenzyme Q10 supplementation in patients with heart failure. They concluded that supplementation with Coenzyme Q10 may represent a safe therapeutic option for patients with heart failure.
Numerous small trials with CoQ10 supplementation in heart failure populations extending back over 30 years have shown adjuvant therapy with Coenzyme Q10 to have beneficial heart health effects such as improvement in NYHA functional class, in the 6-minute walk, in stroke index, in cardiac index score, and in ejection fraction. One large randomized controlled trial, Morisco (1993), showed decreased hospital admissions, decreased episodes of pulmonary edema, and decreased episodes of cardiac asthma. The second large randomized controlled trial, Dr. Mortensen’s Q-Symbio trial (2014), demonstrated a reduction in major adverse cardiovascular events.
The current literature suggests that supplementation with Coenzyme Q10 is relatively safe with very few drug interactions and side effects. Moreover, it is already widely available as an over-the-counter supplement.
The legacy of Dr. Karl Folkers: extensive clinical research into the safety and the effects of Coenzyme Q10
Dr. Folkers set the standard for clinical research into the safety and the effects of Coenzyme Q10 supplementation. He assisted and collaborated with Dr. Svend Aage Mortensen, Dr. Gian Paolo Littarru, Dr. Per Langsjoen and Dr. Peter H. Langsjoen, Dr. William V. Judy, Mr. Sven Moesgaard and numerous other researchers in getting Coenzyme Q10 clinical trials designed and carried out.
Thanks to the efforts of Dr. Folkers and his followers, we now have results from two large randomized controlled trials – Morisco (1993) and Mortensen (2014) – that show significantly improved symptoms and survival and significantly fewer hospitalizations in heart failure patients with Coenzyme Q10 added on to conventional treatment and compared to placebo treatment. In addition, we have the results of many smaller studies that confirm a positive health effect of Coenzyme Q10 supplementation for heart failure patients, e.g. Langsjoen (1985), Munkholm (1999), Keogh (2003), Berman (2004), Kocharian (2009), Pourmoghaddas (2014).
We have three meta-analyses – Soja (1997), Sander (2006), and Fotino (2013) – and two systematic literature reviews – Rosenfeldt (2003) and DiNicolantonio (2015) – that show improvements in various parameters such as NYHA functional class, ejection fraction, stroke volume, and cardiac output without side effects.
Clinical research results show that statin medication therapy reduces the body’s bio-synthesis of Coenzyme Q10 and that supplementation with Coenzyme Q10 is important for patients taking statin medications – Folkers (1990, Langsjoen (2007), Pourmoghaddas (2014).
American and Australian studies have shown that supplementation with Coenzyme Q10 prior to and following heart surgery reduce the number and severity of complications and can reduce the length of the hospital stay [39,55].
The meta-analyses and systematic literature reviews show that Coenzyme Q10 as an adjuvant treatment in chronic heart failure is exceptionally safe and well-tolerated.
Last but not least, we have the results of the KiSel-10 study results showing significantly reduced cardiovascular mortality in healthy elderly Swedish citizens after four years of combined selenium and Coenzyme Q10 supplementation.
Thanks to the clinical research initiated by Dr. Folkers, we now understand much more about the mechanisms by which Coenzyme Q10 improves the working of the failing heart. Coenzyme Q10 supplementation improves the cardiac ATP production, serves as a powerful antioxidant, and helps to correct endothelial dysfunction.
References
1. ACCF/AHA Task Force Report. (2013). 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Journal of The American College of Cardiology, 62(16), e147-e239.
2. Adarsh, K., Kaur, H., & Mohan, V. (2008). Coenzyme Q10 (CoQ10) in isolated diastolic heart failure in hypertrophic cardiomyopathy (HCM). Biofactors (Oxford, England), 32(1-4), 145-149.
3. Alehagen, U., Johansson, P., Björnstedt, M., Rosén, A., & Dahlström, U. (2013). Cardiovascular mortality and N-terminal-proBNP reduced after combined selenium and coenzyme Q10 supplementation: a 5-year prospective randomized double-blind placebo-controlled trial among elderly Swedish citizens. International Journal of Cardiology, 167(5), 1860-1866.
4. Alehagen, U., Aaseth, J., & Johansson, P. (2015). Reduced Cardiovascular Mortality 10 Years after Supplementation with Selenium and Coenzyme Q10 for Four Years: Follow-Up Results of a Prospective Randomized Double-Blind Placebo-Controlled Trial in Elderly Citizens. Plos One, 10(12), e0141641.
5. Alehagen, U., & Aaseth, J. (2015). Selenium and coenzyme Q10 interrelationship in cardiovascular diseases--A clinician's point of view. Journal of Trace Elements in Medicine and Biology, 31157-162.
6. Alehagen, U., Lindahl, T. L., Aaseth, J., Svensson, E., & Johansson, P. (2015). Levels of sP-selectin and hs-CRP decrease with dietary intervention with selenium and Coenzyme Q10 combined: A Secondary analysis of a randomized clinical trial. Plos ONE, 10(9), 1-16.
7. Alehagen, U., Aaseth, J., & Johansson, P. (2015). Less increase of copeptin and MR-proADM due to intervention with selenium and coenzyme Q10 combined: Results from a 4-year prospective randomized double-blind placebo-controlled trial among elderly Swedish citizens. Biofactors (Oxford, England), 41(6), 443-452.
8. Baggio, E., Gandini, R., Plancher, A. C., Passeri, M., & Carmosino, G. (1993). Italian multicenter study on the safety and efficacy of coenzyme Q10 as adjunctive therapy in heart failure (interim analysis). The CoQ10 Drug Surveillance Investigators. The Clinical Investigator, 71(8 Suppl), S145-S149.
9. Balercia, G., Mosca, F., Mantero, F., Boscaro, M., Mancini, A., Ricciardo-Lamonica, G., & Littarru, G. (2004). Coenzyme Q10 supplementation in infertile men with idiopathic asthenozoospermia: an open, uncontrolled pilot study. Fertility and Sterility, 81(1), 93-98.
10. Berman, M., Erman, A., Ben-Gal, T., Dvir, D., Georghiou, G. P., Stamler, A., & ... Aravot, D. (2004). Coenzyme Q10 in patients with end-stage heart failure awaiting cardiac transplantation: a randomized, placebo-controlled study. Clinical Cardiology, 27(5), 295-299.
11. Bogsrud, M. P., Langslet, G., Ose, L., Arnesen, K., Sm Stuen, M. C., Malt, U. F., & Retterstøl, K. (2013). No effect of combined coenzyme Q10 and selenium supplementation on atorvastatin-induced myopathy. Scandinavian Cardiovascular Journal: SCJ, 47(2), 80-87
12. Brauner, H., Lüthje, P., Grünler, J., Ekberg, N. R., Dallner, G., Brismar, K., & Brauner, A. (2014). Markers of innate immune activity in patients with type 1 and type 2 diabetes mellitus and the effect of the anti-oxidant coenzyme Q10 on inflammatory activity. Clinical and Experimental Immunology, 177(2), 478-482.
13. Deichmann, R. E., Lavie, C. J., & Dornelles, A. C. (2012). Impact of coenzyme Q-10 on parameters of cardiorespiratory fitness and muscle performance in older athletes taking statins. The Physician and Sports Medicine, 40(4), 88-95.
14. Del Pozo-Cruz, J., Rodríguez-Bies, E., Navas-Enamorado, I., Del Pozo-Cruz, B., Navas, P., & López-Lluch, G. (2014). Relationship between functional capacity and body mass index with plasma coenzyme Q10 and oxidative damage in community-dwelling elderly-people. Experimental Gerontology, 5246-54.
15. Denny N, Chapple I, Matthews JB. (1999). Antioxidant and anti-inflammatory effects of Coenzyme Q10: a preliminary study. Journal of Dental Research, 78, 543.
16. DiNicolantonio JJ, Bhutani J, McCarty MF, O'Keefe JH. (2015). Coenzyme Q10 for the treatment of heart failure: a review of the literature. Open Heart, 19;2(1):e000326.
17. Engelsen, J., Nielsen, J. D., & Winther, K. (2002). Effect of coenzyme Q10 and Ginkgo biloba on warfarin dosage in stable, long-term warfarin treated outpatients. A randomised, double blind, placebo-crossover trial. Thrombosis And Haemostasis, 87(6), 1075-1076.
18. Ernster L. (1977) Facts and ideas about the function of Coenzyme Q10 in the Mitochondria. In: Folkers K., Yamamura Y.(eds.) Biomedical and Clinical Aspects of Coenzyme Q. Amsterdam: Elsevier, 15-8.
19. Eriksson, J. G., Forsén, T. J., Mortensen, S. A., & Rohde, M. (1999). The effect of coenzyme Q10 administration on metabolic control in patients with type 2 diabetes mellitus. Biofactors (Oxford, England), 9(2-4), 315-318.
20. Fedacko, J., Pella, D., Fedackova, P., Hänninen, O., Tuomainen, P., Jarcuska, P., & Littarru, G. P. (2013). Coenzyme Q10 and selenium in statin-associated myopathy treatment. Canadian Journal of Physiology and Pharmacology, 91(2), 165-170.
21. Folkers K, Littarru GP, Ho L, Runge TM, Havanonda S, & Cooley D. (1970). Evidence for a deficiency of coenzyme Q10 in human heart disease. International Journal of Vitamin Research 40(3):380-90.
22. Folkers, K, Vadhanavikit, S, Mortensen, SA. (1985). Biochemical rationale and myocardial tissue data on the effective therapy of cardiomyopathy with Coenzyme Q10. Proc. Natl. Acad. Sci. USA. 82, 901-904.
23. Folkers, K., Langsjoen, P., Willis, R., Richardson, P., Xia, L. J., Ye, C. Q., & Tamagawa, H. (1990). Lovastatin decreases coenzyme Q levels in humans. Proceedings of The National Academy of Sciences of The United States of America, 87(22), 8931-8934.
24. Folkers K, Osterborg A, Nylander M, Morita M, Mellstedt H. (1997). Activities of vitamin Q10 in animal models and a serious deficiency in patients with cancer. Biochem Biophys Res Commun. 234(2):296-299.
25. Folkers, K., Moesgaard, S., & Morita, M. (1994). A one year bioavailability study of coenzyme Q10 with 3 months withdrawal period. Molecular Aspects of Medicine, 15 Suppls281-s285.
26. Fotino, A. D., Thompson-Paul, A. M., & Bazzano, L. A. (2013). Effect of coenzyme Q₁₀ supplementation on heart failure: a meta-analysis. The American Journal of Clinical Nutrition, 97(2), 268-275.
27. Golomb, B. A., Allison, M., Koperski, S., Koslik, H. J., Devaraj, S., & Ritchie, J. B. (2014). Coenzyme Q10 benefits symptoms in Gulf War veterans: results of a randomized double-blind study. Neural Computation, 26(11), 2594-2651.
28. Hathcock JN, Shao A. (2006). Risk assessment for coenzyme Q10 (Ubiquinone). Regul Toxicol Pharmacol. 45(3):282-288.
29. Henriksen, J. E., Andersen, C. B., Hother-Nielsen, O., Vaag, A., Mortensen, S. A., & Beck-Nielsen, H. (1999). Impact of ubiquinone (Coenzyme Q10) treatment on glycemic control, insulin requirement and well-being in patients with Type 1 diabetes mellitus. Diabetic Medicine: A Journal of the British Diabetic Association, 16(4), 312-318.
30. Hidaka, T., Fujii, K., Funahashi, I., Fukutomi, N., & Hosoe, K. (2008). Safety assessment of Coenzyme Q10 (CoQ10). Biofactors (Oxford, England), 32(1-4), 199-208.
31. Hodges, S., Hertz, N., Lockwood, K., & Lister, R. (1999). CoQ10: could it have a role in cancer management? Biofactors (Oxford, England), 9(2-4), 365-370.
32. Huntington Study Group. (2001). A randomized, placebo-controlled trial of coenzyme Q10 and remacemide in Huntington's disease. Neurology. 57(3):397-404.
33. Ikematsu, H., Nakamura, K., Harashima, S., Fujii, K., & Fukutomi, N. (2006). Safety assessment of coenzyme Q10 (Kaneka Q10) in healthy subjects: a double-blind, randomized, placebo-controlled trial. Regulatory Toxicology and Pharmacology, 44(3), 212-218.
34. Johansson, P., Dahlström, Ö., Dahlström, U., & Alehagen, U. (2013). Effect of selenium and Q10 on the cardiac biomarker NT-proBNP. Scandinavian Cardiovascular Journal: SCJ, 47(5), 281-288.
35. Johansson, P., Dahlström, Ö., Dahlström, U., & Alehagen, U. (2015). Improved health-related quality of life, and more days out of hospital with supplementation with selenium and Coenzyme Q10 combined. Results from a double blind, placebo-controlled prospective study. The Journal of Nutrition, Health & Aging, 19(9), 870-877.
36. Judy, WV, Hall, JH, and & Folkers, K. (1991). Coenzyme Q10 withdrawal – clinical relapse in congestive heart failure, in: Biomedical and Clinical Aspects of Coenzyme Q, (Vol. 6), K. Folkers, G.P. Littarru and T. Yamagami, eds. Amsterdam: Elsevier. 283–290.
37. Judy, WV, Folkers, K & Hall, JH. (1991). Improved long-term survival in coenzyme Q10 treated congestive heart failure patients compared to conventionally treated patients, in: Biomedical and Clinical Aspects of Coenzyme Q, (Vol. 6), K. Folkers, G.P. Littarru and T. Yamagami, eds. Amsterdam: Elsevier. 291–298.
38. Judy, WV, Hall, JH, Toth PD, & Folkers, K. (1984). Myocardial effects of co-enzyme Q10 in primary heart failure, in: Biomedical and Clinical Aspects of Coenzyme Q, (Vol. 4), K. Folkers and Y. Yamamura, eds. Amsterdam: Elsevier. 353–367.
39. Judy, WV, Stogsdill, WW, & Folkers, K. (1993). Myocardial preservation by therapy with Coenzyme Q10 during heart surgery. Clinical Investigator, 71(8 Suppl):S155-61.
40. Judy, WV, Hall, JH, Dugan, W, Toth, PD, Folkers, K. (1984). Coenzyme Q10 reduction of Adriamycin cardiotoxicity. In: Folkers K, Yamamura Y, eds. Biomedical and Clinical Aspects of Coenzyme Q. Vol 4. Amsterdam: Elsevier/North-Holland Biomedical Press; 231-241.
41. Judy, W.V., Stogsdill, W.W., Judy, D.S., & Judy, J.S. (2007). Coenzyme Q10: Facts or Fabrications? Natural Products Insider. Retrieved from http://www.zmc-usa.com/docs/CoQ10_Facts_or_Fabrications.pdf.
42. Kalen A, Appelkvist EL, Dallner G. Age-related changes in the lipid compositions of rat and human tissues. Lipids. 1989;24(7):579–584.
43. Keogh, A., Fenton, S., Leslie, C., Aboyoun, C., Macdonald, P., Zhao, Y. C., & Rosenfeldt, F. (2003). Randomised double-blind, placebo-controlled trial of coenzyme Q, therapy in class II and III systolic heart failure. Heart, Lung & Circulation, 12(3), 135-141.
44. Khatta, M., Alexander, B. S., Krichten, C. M., Fisher, M. L., Freudenberger, R., Robinson, S. W., & Gottlieb, S. S. (2000). The effect of coenzyme Q10 in patients with congestive heart failure. Annals of Internal Medicine, 132(8), 636-640.
45. Kocharian, A., Shabanian, R., Rafiei-Khorgami, M., Kiani, A., & Heidari-Bateni, G. (2009). Coenzyme Q10 improves diastolic function in children with idiopathic dilated cardiomyopathy. Cardiology in the Young, 19(5), 501-506.
46. Kuklinski, B., Weissenbacher, E., & Fähnrich, A. (1994). Coenzyme Q10 and antioxidants in acute myocardial infarction. Molecular Aspects of Medicine, 15 Suppls143-s147.
47. Langsjoen, PH., Vadhanavikit, S., & Folkers, K. (1985). Effective treatment with coenzyme Q10 of patients with chronic myocardial disease. Drugs Under Experimental and Clinical Research, 11(8), 577-579.
48. Langsjoen, PH., Vadhanavikit, S., & Folkers, K. (1985). Response of patients in classes III and IV of cardiomyopathy to therapy in a blind and crossover trial with coenzyme Q10. Proceedings of The National Academy Of Sciences Of The United States Of America, 82(12), 4240-4244.
49. Langsjoen, P. H., Folkers, K., Lyson, K., Muratsu, K., Lyson, T., & Langsjoen, P. (1988). Effective and safe therapy with coenzyme Q10 for cardiomyopathy. Klinische Wochenschrift, 66(13), 583-590.
50. Langsjoen, P, Langsjoen, PH, & Folkers, K. (1990). A six-year clinical study of therapy of cardiomyopathy with coenzyme Q10, International Journal of Tissue Reactions 12, 169–171.
51. Langsjoen, P. H., Langsjoen, J. O., Langsjoen, A. M., & Lucas, L. A. (2005). Treatment of statin adverse effects with supplemental Coenzyme Q10 and statin drug discontinuation. Biofactors (Oxford, England), 25(1-4), 147-152.
52. Langsjoen, H., Langsjoen, P., Langsjoen, P., Willis, R., & Folkers, K. (1994). Usefulness of coenzyme Q10 in clinical cardiology: a long-term study. Molecular Aspects of Medicine, 15 Suppls165-s175.
53. Lee, B., Huang, Y., Chen, S., & Lin, P. (2012). Coenzyme Q10 supplementation reduces oxidative stress and increases antioxidant enzyme activity in patients with coronary artery disease. Nutrition (Burbank, Los Angeles County, Calif.), 28(3), 250-255.
54. Lee, B., Tseng, Y., Yen, C., & Lin, P. (2013). Effects of coenzyme Q10 supplementation (300 mg/day) on antioxidation and anti-inflammation in coronary artery disease patients during statins therapy: a randomized, placebo-controlled trial. Nutrition Journal, 12(1), 142.
55. Leong, J., van der Merwe, J., Pepe, S., Bailey, M., Perkins, A., Lymbury, R., & Rosenfeldt, F. (2010). Perioperative metabolic therapy improves redox status and outcomes in cardiac surgery patients: a randomised trial. Heart, Lung & Circulation, 19(10), 584-591.
56. Lewin, A. & Lavon, H. (1997). The Effect of Coenzyme Q10 on sperm motility and function. Molecular Aspects of Medicine, 18 (Supplement), s213-s219.
57. Littarru, G. P., & Langsjoen, P. (2007). Coenzyme Q10 and statins: biochemical and clinical implications. Mitochondrion, 7 SupplS168-S174.
58. Lockwood, K., Moesgaard, S., Hanioka, T., & Folkers, K. (1994). Apparent partial remission of breast cancer in 'high risk' patients supplemented with nutritional antioxidants, essential fatty acids and coenzyme Q10. Molecular Aspects Of Medicine, 15 Suppls231-s240.
59. Lockwood, K., Moesgaard, S., Yamamoto, T., & Folkers, K. (1995). Progress on therapy of breast cancer with vitamin Q10 and the regression of metastases. Biochemical And Biophysical Research Communications, 212(1), 172-177.
60. McMurray, J. V., Dunselman, P., Wedel, H., Cleland, J. F., Lindberg, M., Hjalmarson, A., & Wikstrand, J. (2010). Coenzyme Q10, rosuvastatin, and clinical outcomes in heart failure: a pre-specified substudy of CORONA (controlled rosuvastatin multinational study in heart failure). Journal of the American College Of Cardiology, 56(15), 1196-1204.
61. Madmani, M. E., Yusuf Solaiman, A., Tamr Agha, K., Madmani, Y., Shahrour, Y., Essali, A., & Kadro, W. (2014). Coenzyme Q10 for heart failure. The Cochrane Database of Systematic Reviews, 6CD008684.
62. Molyneux, S. L., Florkowski, C. M., George, P. M., Pilbrow, A. P., Frampton, C. M., Lever, M., & Richards, A. M. (2008). Coenzyme Q10: an independent predictor of mortality in chronic heart failure. Journal of The American College Of Cardiology, 52(18), 1435-1441.
63. Mohr, D., Bowry, V. W., & Stocker, R. (1992). Dietary supplementation with coenzyme Q10 results in increased levels of ubiquinol-10 within circulating lipoproteins and increased resistance of human low-density lipoprotein to the initiation of lipid peroxidation. Biochimica et Biophysica Acta, 1126(3), 247-254.
64. Morisco, C., Trimarco, B., & Condorelli, M. (1993). Effect of coenzyme Q10 therapy in patients with congestive heart failure: a long-term multicenter randomized study. The Clinical Investigator, 71(8 Suppl), S134-S136.
65. Mortensen, SA., Vadhanavikit, S., Muratsu, K., & Folkers, K. (1990). Coenzyme Q10: clinical benefits with biochemical correlates suggesting a scientific breakthrough in the management of chronic heart failure. International Journal of Tissue Reactions, 12(3), 155-162.
66. Mortensen, SA., Vadhanavikit, S., Baandrup, U., & Folkers, K. (1985). Long-term coenzyme Q10 therapy: a major advance in the management of resistant myocardial failure. Drugs Under Experimental and Clinical Research, 11(8), 581-593.
67. Mortensen, S. A., Leth, A., Agner, E., & Rohde, M. (1997). Dose-related decrease of serum coenzyme Q10 during treatment with HMG-CoA reductase inhibitors. Molecular Aspects of Medicine, 18 SupplS137-S144.
68. Mortensen, S. A. (2003). Overview on coenzyme Q10 as adjunctive therapy in chronic heart failure. Rationale, design and end-points of "Q-Symbio"--a multinational trial. Biofactors (Oxford, England), 18(1-4), 79-89.
69. Mortensen, S. A. (2011). Low coenzyme Q₁₀ levels and the outcome of statin treatment in heart failure. Journal of The American College of Cardiology, 57(14), 1569; author reply 1569.
70. Mortensen, S. A., Rosenfeldt, F., Kumar, A., Dolliner, P., Filipiak, K. J., Pella, D., & Littarru, G. P. (2014). The effect of coenzyme Q10 on morbidity and mortality in chronic heart failure: results from Q-SYMBIO: a randomized double-blind trial. JACC. Heart Failure, 2(6), 641-649.
71. Mortensen, S. A. (2015). Coenzyme Q10: will this natural substance become a guideline-directed adjunctive therapy in heart failure? JACC. Heart Failure, 3(3), 270-271.
72. Mortensen, S. A., & Mortensen, A. L. (2014). The mitochondria in heart failure: a target for coenzyme Q10 therapy? Clinical Pharmacology and Therapeutics, 96(6), 645-647.
73. Munkholm, H., Hansen, H. H., & Rasmussen, K. (1999). Coenzyme Q10 treatment in serious heart failure. Biofactors (Oxford, England), 9(2-4), 285-289.
74. Nylander, M., Weiner, J., & Nordlund, M. (1996). A double-blind clinical dose-response study on effects of CoQ10 on gingival bleeding/periodontal disease in ordinary people. Helsinki, Finland: 7th International Symposium on Trends in Biomedicine in Finland, Suppl 8, 1-7.
75. Okuyama, H., Langsjoen, P. H., Hamazaki, T., Ogushi, Y., Hama, R., Kobayashi, T., & Uchino, H. (2015). Statins stimulate atherosclerosis and heart failure: pharmacological mechanisms. Expert Review of Clinical Pharmacology, 8(2), 189-199.
76. Olson, RE. (2001). Karl August Folkers (1906–1997). Journal of Nutrition 131 (9): 2227–30.
77. Pourmoghaddas, M., Rabbani, M., Shahabi, J., Garakyaraghi, M., Khanjani, R., & Hedayat, P. (2014). Combination of atorvastatin/coenzyme Q10 as adjunctive treatment in congestive heart failure: A double-blind randomized placebo-controlled clinical trial. ARYA Atherosclerosis, 10(1), 1-5.
78. Rosenfeldt, F., Hilton, D., Pepe, S., & Krum, H. (2003). Systematic review of effect of coenzyme Q10 in physical exercise, hypertension and heart failure. Biofactors (Oxford, England), 18(1-4), 91-100.
79. Sander, S., Coleman, C. I., Patel, A. A., Kluger, J., & White, C. M. (2006). The impact of coenzyme Q10 on systolic function in patients with chronic heart failure. Journal of Cardiac Failure, 12(6), 464-472.
80. Sharma, A., Fonarow, G. C., Butler, J., Ezekowitz, J. A., & Felker, G. M. (2016). Coenzyme Q10 and Heart Failure: A State-of-the-Art Review. Circulation. Heart Failure, 9(4), e002639.
81. Shive, W. (2002). Karl August Folkers, September 1, 1906 – December 9, 1997. Biographical memoirs. National Academy of Sciences (U.S.) 81: 100–14.
82. Shults CW, Oakes D, Kieburtz K. (2002). Effects of coenzyme Q10 in early Parkinson disease: evidence of slowing of the functional decline. Arch Neurol. 59(10):1541-1550.
83. Shults, C. W., Flint Beal, M., Song, D., & Fontaine, D. (2004). Pilot trial of high dosages of coenzyme Q10 in patients with Parkinson's disease. Experimental Neurology, 188(2), 491-494.
84. Sinatra, S. T. in response to Gottlieb, S., Khatta, M., & Fisher, M. (2000). Coenzyme Q10 and Congestive Heart Failure. Annals of Internal Medicine, 133(9), 745-746.
85. Singh, R. B., Wander, G. S., Rastogi, A., Shukla, P. K., Mittal, A., Sharma, J. P., & Chopra, R. K. (1998). Randomized, double-blind placebo-controlled trial of coenzyme Q10 in patients with acute myocardial infarction. Cardiovascular Drugs and Therapy, 12(4), 347-353.
86. Singh, R. B., Niaz, M. A., Kumar, A., Sindberg, C. D., Moesgaard, S., & Littarru, G. P. (2005). Effect on absorption and oxidative stress of different oral Coenzyme Q10 dosages and intake strategy in healthy men. Biofactors (Oxford, England), 25(1-4), 219-224.
87. Soja, A. M., & Mortensen, S. A. (1997). Treatment of congestive heart failure with coenzyme Q10 illuminated by meta-analyses of clinical trials. Molecular Aspects Of Medicine, 18 SupplS159-S168.
88. Weber, C., Sejersgård Jakobsen, T., Mortensen, S. A., Paulsen, G., & Hølmer, G. (1994). Antioxidative effect of dietary coenzyme Q10 in human blood plasma. International Journal for Vitamin and Nutrition Research 64(4), 311-315.
89. Weber, C., & Bysted, A. H. (1997). Intestinal absorption of Coenzyme Q10 administered in a meal or as capsules to healthy subjects. Nutrition Research, 17, 6, 941-945.
90. Weis, M., Mortensen, S. A., Rassing, M. R., Møller-Sonnergaard, J., Poulsen, G., & Rasmussen, S. N. (1994). Bioavailability of four oral coenzyme Q10 formulations in healthy volunteers. Molecular Aspects of Medicine, 15 Suppls273-s280.
91. Ylikoski, T., Piirainen, J., Hanninen, O., & Penttinen, J. (1997). The effect of coenzyme Q10 on the exercise performance of cross-country skiers. Molecular Aspects of Medicine, 18 SupplS283-S290.
92. Yokoyama, H., Lingle, D. M., Crestanello, J. A., Kamelgard, J., Kott, B. R., Momeni, R., & Whitman, G. J. (1996). Coenzyme Q10 protects coronary endothelial function from ischemia reperfusion injury via an antioxidant effect. Surgery, 120(2), 189-196.
93. Zhou, Q., Zhou, S., & Chan, E. (2005). Effect of coenzyme Q10 on warfarin hydroxylation in rat and human liver microsomes. Current Drug Metabolism, 6(2), 67-81.
94. Zita, C., Overvad, K., Mortensen, S. A., Sindberg, C. D., Moesgaard, S., & Hunter, D. A. (2003). Serum coenzyme Q10 concentrations in healthy men supplemented with 30 mg or 100 mg coenzyme Q10 for two months in a randomised controlled study. Biofactors (Oxford, England), 18(1-4), 185-193.

