The vital nutrient that most of us are lacking
Many people have never heard of selenium, yet this microscopic mineral could be more vital to our health than any other nutrient. Studies link low selenium intake with an increased risk of cancer and other problems. Unfortunately, people in many countries fail to get enough selenium to optimize their health. This is partly due to the fact that the agricultural soil in many parts of the world is low in selenium, and the problem is only made worse by unbalanced diets, veganism, and gluten intolerance (wheat is a selenium source).
Somewhat surprisingly, the selenium content in organically farmed produce is often lower than crops, meat, and dairy from conventional farms, which is because organic farming prohibits selenium-enrichment of agricultural fertilizers. This was otherwise what the Finnish government decided to introduce back in 1985 to improve the critically low selenium status of the Finns –and it worked.
Today, more and more experts call for selenium supplementation as a useful measure for improving global health. In fact, farmers have fed supplementary selenium to livestock for decades as a natural way of preventing muscle and joint problems, heart disease, and low fertility in the animals
In his book “History of Selenium”, Richard Morrill takes a closer look at some of the studies that link improved selenium status to lower cancer rates and fewer cardiovascular diseases. Science is finding out more and more about the health benefits of this fascinating nutrient. The big challenge is getting enough to stay healthy.
The Vital Nutrient That May Save Lives
Selenium, element number 34 in the periodic table, is a curious trace element in many respects. It is an absolutely vital component of the selenium-dependent proteins that have several biological functions in human health, including an antioxidant function. Although it is very important for human health, selenium is not utilized as a free element in the human body. Furthermore, selenium is relatively rare in nature, and it is very unevenly distributed in the soils of the earth. Consequently, it is very unevenly distributed in food sources in the world. All of these characteristics make it a very unusual micronutrient but no less important.
Selenium a little known micronutrient
Even though selenium is critically important for good human health, its nutritional value is little known. It is important both for the prevention of selenium-related deficiency diseases and for its health promoting biological functions as part of the amino acid selenocysteine that is incorporated into the selenoproteins. It seems strange that such an important nutrient should be so scarce in the world and so little known. We all need it, and, for many of us, it is difficult to get enough on a daily basis.
Selenium-poor soils in much of the world
Large parts of Europe and the Middle East, Africa, Asia, and New Zealand are known to have low selenium concentrations in the soil and in the locally grown food. Similarly, large parts of certain regions of the United States – the Northeast, the Southeast, the Northwest, and the Southwest – have lower than average selenium concentrations in the soil and in the locally produced foodstuffs.
Selenium from the diet
In varying degrees from region to region of the world, people get their daily selenium from eating breads, cereals, eggs, fish, meat, and poultry. Many people do not get adequate amounts of selenium in their diets and need a daily selenium supplement. To the extent that people eliminate fish and meats and/or eggs and dairy products from their diet, admittedly for principled reasons, to that extent they need a daily dietary supplement even more.
Selenium not synthesized in the human body
The human body does not synthesize selenium. Dietary and supplemental selenium is required for the optimal functioning of the selenium-dependent enzymes known as selenoproteins.
Selenium deficiency a real possibility in parts of the world
Selenium deficiency diseases are known in both animals and humans. Keshan disease is a potentially fatal heart muscle disease that was first diagnosed in selenium-poor regions of China. Kashin-Beck disease is a chronic bone disease that occurs in selenium-poor regions of China, Korea, Siberia, and Tibet. White-muscle disease has been prevalent in animals, especially in ruminants, in regions in which the soil and the feedstuffs are lacking in selenium.
At-risk groups in danger of getting too little dietary selenium
Above and beyond the inadequacy of the dietary intake of selenium that results when people live in regions with selenium-poor soils and selenium-poor foods, there are groups of people who will need supplementary selenium regardless of where they live and grow or buy their food.
In many cases, people who choose to be careful about what they eat will need a good selenium supplement. For example, people who eat organically produced foods, people who are vegetarians or vegans, and people who suffer from gluten intolerance are all people who will need supplemental selenium.
Similarly, people who have various medical conditions will need extra selenium beyond what they get in their diet. These are people who are on kidney dialysis, people who are HIV-infected, people who use above-average amounts of alcohol, and people who are smokers.
People who are elderly and eat less and women who are pregnant or who are breast-feeding may well need a selenium supplement.
Selenium as a component of selenoproteins
Selenium in the form of selenocysteine, the 21st amino acid, is incorporated preferentially into the selenoproteins. The selenoproteins play a protective role in human reproduction, thyroid hormone metabolism, DNA synthesis and maintenance, oxidative stress, viral infections, and degenerative diseases such as cancer and heart disease.
Official government awareness of selenium’s importance
Governments around the world take the problem of inadequate selenium intakes seriously. Official proclamations from various governments attest to the importance of dietary and supplemental selenium.
US Food and Drug Administration
In recognition of the importance of adequate selenium intakes, the US Food and Drug Administration permits the following claims on packages of selenium supplements:
"Selenium may reduce the risk of certain cancers. Some scientific evidence suggests that consumption of selenium may reduce the risk of certain forms of cancer. However, FDA has determined that this evidence is limited and not conclusive."
"Selenium may produce anti-carcinogenic effects in the body. Some scientific evidence suggests that consumption of selenium may produce anti-carcinogenic effects in the body. However, FDA has determined that this evidence is limited and not conclusive."
The European Food Safety Authority
The European Food Safety Authority has issued a statement to the effect that “a cause and effect relationship has been established between the dietary intake of selenium and protection of DNA, proteins, and lipids from oxidative damage, normal immune function, normal thyroid function, and normal spermatogenesis.”
The Government of Finland
In 1985, the Finnish government required, by law, the addition of selenium to agricultural fertilizers. The stated justification for the law was the knowledge that selenium deficiency increases the risk of cancer and heart disease. In 2005, the MTT Agrifood Research Finland Group published research results showing that, as a result of the addition of selenium to fertilizers, the selenium intakes of animals and healthy people in Finland was safe and adequate.
Interestingly, the only Finns who were not showing a safe and adequate selenium intake by 2005 were the people who consumed food from the unfertilized organic farming and gardening plots. Clearly, people need a daily selenium supplement if they make the wise choice to eat organically grown foods.
Small fraction of available selenium used for food production
Of the total world production of selenium, which is not large, most of the recovered selenium is used for industrial purposes. Perhaps 10 percent of the total selenium available is used for animal feed fertilizer. Much less than 10 percent is used for nutritional supplements.
Not enough selenium for extensive fertilization projects
The Finnish experiment involving the addition of selenium to the fertilization spread onto selenium-poor soils has been shown to be a success. Unfortunately, there is not enough available selenium for all of the regions of the world that lack selenium in the soil and in the locally grown food to copy the Finnish experiment and fertilize all of these soils with selenium.
Less selenium from good intentions
Ironically, the movement towards cleaner forms of energy production has led to decreasing deposits of selenium in the soil. Studies reveal that the highest concentrations of the deposit of airborne selenium particulate matter occurred between 1940 – 1970 during the period of intensive coal use. With the shift to more use of energy sources such as nuclear power, oil and natural gas, and wind and solar power in the years since 1970, the deposits of selenium particulate matter have declined. The result has been a decline in the selenium concentrations in grasses, grains, and other vegetation.
In the same manner, the movement in recent years from conventional farming to organic farming – a laudable movement in every conceivable way --has also led to a decline in the availability of selenium in the diet. A Danish study has shown, for example, that the selenium concentration is much lower in milk from organic farms than in the milk from conventional farms.
Health benefits of selenium supplementation seen in randomized controlled studies
Heart disease study
Randomized controlled trials have shown significant health benefits associated with taking a daily selenium-enriched yeast supplement.
In the KiSel-10 study, the quartile of study participants that had the highest serum selenium status had significantly lower mortality rates than the quartile that had the lowest serum selenium status. This effect continued to be seen 10 years after the initiation of the four-year period of daily supplementation with 200 micrograms [Alehagen 2015].
Cancer studies
In the Nutritional Prevention of Cancer study, patients treated daily with 200 micrograms for 4.5 years had significantly lower rates of colon, lung, prostate, and total cancer [Clark 1996]. Similarly, in the Su.Vi.Max study, daily supplementation with 100 micrograms of selenium and other antioxidants resulted in 31% lower risk of cancer incidence and 37% lower all-cause risk of cancer mortality in men [Hercberg 2010]. And, in the Nutritional Intervention study, daily supplementation with 50 micrograms of selenium together with the antioxidants beta-carotene and vitamin E significantly reduced cancer mortality rates [Blot 1993].
Cross-sectional studies
Two large cross-sectional studies revealed an association between selenium status and the risk of prostate cancer. In the Danish Diet, Cancer and Health survey of 27,179 male participants, 80% of Danish men had too little selenium in the blood to ensure that the selenium-dependent enzyme selenoprotein P functions optimally. Selenoprotein P helps to protect men against the development of prostate cancer [Outzen 2016].
In the Netherlands Cohort Study, researchers at the Maastricht Medical Centre found that men with high selenium levels in their toenails had a significantly better protection against prostate cancer than men with low selenium status. The researchers’ conclusion was based on an analysis of toenail samples from 79% of the 58,279 men aged 55-66 years. The quartile with the lowest selenium status had the highest rate of prostate cancer [Geybels 2013].
Need to conserve selenium
Because adequate intakes of selenium are so important for the good health of humans and animals, our uses of selenium need to be managed carefully. Selenium will continue to be an essential micronutrient for humans and animals, the more so as the human population continues to grow. Selenium will need to be stockpiled for use as a nutrient for future generations [Haug 2007].
The nutritional importance of dietary and supplemental selenium is that selenium is needed for at least three primary purposes:
- To avoid selenium deficiency conditions
- To replenish the antioxidant selenoproteins and reduce oxidative cell damage
- To reduce the risk of cancer, cardiovascular disease, thyroid disorders, and neurodegenerative disorders
Structure and purpose of this essay
In this essay, we provide a history of selenium supplementation in human health. We focus on the clinical trials that link improved selenium status to lower risk of cancer incidence and mortality, lower risk of heart disease, good thyroid function, protection against the toxic effects of heavy metals, protection against viral infections, and protection against oxidative damage.
The basic structure of this history is topical by medical condition, and, within the topic, chronological to show the development of a knowledge base about selenium supplementation over time. First, though, we provide background information about the element selenium and the various compounds in which it occurs in the soil, in food and in supplements, and in the body.
Selenium discovered by Berzelius
The element selenium was discovered in 1817 more or less by accident by Jöns Jakob Berzelius and Johan Gottlieb Gahn. Professor J. E. Oldfield said once that he doubted that Berzelius, as brilliant as he was, could have imagined that selenium would eventually prove to be an essential micronutrient with anti-carcinogenic properties.
Selenium has the following properties:
- It has six electrons in its outermost shell.
- It is a non-metal that can combine with both metals and non-metals.
- It can form both organic and inorganic compounds.
- It has six naturally occurring isotopes.
Its chemistry is such that it can serve as both an oxidizing agent and as a reducing agent.
In countries such as England and Norway, cereals were previously an important source of selenium because these countries imported selenium-rich grains from the United States and Canada. In the American food tables, one kilogram of wheat was declared to contain 707 micrograms of selenium. Wheat grown in Norway, however, contains only 20 micrograms of selenium per kilogram.
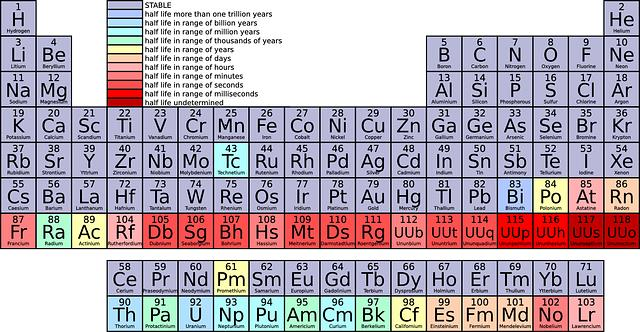
Illustration. The periodic table. Selenium, abbreviated Se, has the atomic number 34, which is the number of protons in its nucleus. In the periodic table, selenium belongs in the Sulfur group, in the third column from the right, just below sulfur and just above tellurium.
Selenium: rare and unevenly distributed on Earth
Both in the body and in nature, selenium is a rare element. Moreover, in the earth’s crust, it is unevenly distributed. It cannot be emphasized enough that selenium status in humans varies considerably relative to where a person lives and gets his food.
Selenium in food
The amount of selenium in the foods that people typically eat is quite low. Brazil nuts are known to have a high content of selenium, but not many people eat Brazil nuts regularly. The amount of selenium in cereals and other grains varies considerably from region to region depending upon how much selenium there is in the soil. Consequently, individual intakes of selenium vary widely from region to region and from diet to diet.
Selenomethionine is the major seleno-compound found cereal grains, in legumes, in animals, and in selenium enriched yeast nutritional supplements. In animals, much of the selenomethionine is converted to the amino acid selenocysteine. The percentage of selenomethionine in high selenium yeast supplements varies according to the production condition.
The major inorganic seleno-compound found in animals and plants is selenate. The major organic seleno-compound found in selenium-rich plants such as broccoli, garlic, leeks, and onions is selenium-methylselenocysteine [Whanger 2002].

Illustration: Sven Moesgaard and Eli Wallin (right), the founders in 1984 of the firm Pharma Nord in Denmark. Their first products were the Bio-Selenium and the Bio-Selenium + Zinc preparations (pictured on the table), followed shortly thereafter by the Coenzyme Q10 preparation Bio-Quinone Q10. Both men were eager to test their preparations in randomized controlled clinical trials. Both men wanted to produce nutritional supplements that they themselves would want to take.
Selenium supplementation
Given the current state of our knowledge of the functions of selenium and selenoproteins (about which more, later in this paper), the optimal total adult selenium intake seems to be in the range of 250 to 300 micrograms per day. Depending on where one lives and what kind of diet one eats, reaching that daily intake level would require nutritional supplementation of 100 to 200 micrograms per day [Schrauzer 2009].
The most appropriate form of the nutritional selenium supplement is the selenium- enriched yeast form, also called high selenium yeast. This form contains selenium primarily in the form of L-selenomethionine and contains as many as 30 other minor species of selenium when it has been properly formulated and produced [Larsen & Moesgaard 2004].
Selenium in animal science
Historically, selenium came to the attention first of veterinarians and medical doctors as a toxic substance. Animal and human exposure to high concentrations of selenium resulted in symptoms of illness. In humans, typical signs of selenium toxicity included garlic breath, a metal taste in the mouth, nail discoloration and brittleness, hair loss, muscle or joint pain, nausea, and vomiting [MacFarquhar2010].
Somewhat later, in the period of the late 1950s to the 1970s, researchers discovered that selenium, in trace amounts, is essential to animal and human health. Specifically, the discovery that selenium supplementation could prevent Keshan disease and mitigate Kashin-Beck disease opened researchers’ eyes to the potential benefits of supplementation with selenium [Tolonen 1990].
In 1961, New Zealand, a country with extremely low levels of selenium in its soil, began to make widespread use of selenium to counteract selenium-responsive diseases in domestic animals. Finland, another selenium-poor region of the world, began use selenium to treat muscle diseases in animals and in fertilizers, starting in the 1960s. By 1984, the Finns had begun to add selenium to agricultural fertilizers in an attempt to increase the selenium content of agricultural crops and thereby increase the intake of selenium in the Finnish diet [Tolonen 1990].
Selenium and selenoproteins: nutritional importance
The trace element selenium is an important micronutrient that is a necessary component of the amino acid selenocysteine. Selenocysteine is incorporated into selenoproteins that have diverse functions in human health and disease.
Selenocysteine – the 21st amino acid
Dietary and supplemental selenium is needed to form part of the amino acid selenocysteine, the only amino acid to have a selenium component. Selenocysteine is incorporated into at least 25 selenoproteins that have catalytic and antioxidant functions in the body. The chemical structure of selenocysteine, which is the 21st amino acid, is almost identical with the structure of the amino acid cysteine. The basic difference is that selenium has taken the place of sulfur in the molecule.
Selenocysteine, as a substance, is unstable and cannot be used in selenium supplements [Moesgaard 2001]. It does not exist freely in the cells; it forms a constituent part of the selenoproteins. It is synthesized in a complex manner that requires the bioavailability of selenium from selenomethionine and other selenium compounds. The synthesis of the selenoproteins, in turn, requires the presence of selenocysteine [Rayman 2005].
Supplementation levels and biochemical functions
Selenium supplementation at levels higher than normal dietary intakes – which are quite low in certain regions of the earth – has been shown, in clinical trials, to have beneficial disease fighting and disease mitigating effects [Blot 1993, Clark 1996, Yu 1997].
Margaret P. Rayman of the University of Surrey has suggested that selenium intakes of up to 100 micrograms per day have beneficial effects on selenoprotein antioxidant activity and immune system function while selenium intakes in the range of 200 – 300 micrograms per day have more specific anti-carcinogenic effects [Rayman 2002]. It is difficult, and sometimes impossible, to obtain that much selenium from the diet in many parts of the world.
Known selenoproteins
Selenoproteins are proteins that have incorporated selenium for a specific purpose in the body. One of the primary purposes in human health is the antioxidant activity of the seleno-enzymes. Among the most important selenoproteins, the Linus Pauling Institute lists the selenium-dependent enzymes:
- 5 forms of glutathione peroxidase (8 forms are known)
- 3 forms of thioredoxin reductase
- 3 forms of iodothyronine deiodinase
- 1 form of methionine sulfoxide reductase B1
These selenoproteins, along with selenoprotein P, are the most comprehensively studied to date [Tsuji 2015].
Below, listed more or less in the order of their discovery (P, W H…), is a more complete list of known selenoproteins [Bellinger 2009].
| Selenoprotein P | Selenoprotein 15 |
| Selenoprotein W | Selenoprotein 15 kDa |
| Selenoprotein H | Selenoprotein K |
| Selenoprotein I | Selenoprotein M |
| Selenoprotein R | Selenoprotein T |
| Selenoprotein N | Selenoprotein O |
| Selenoprotein S | Selenoprotein V |
Bio-synthesis of selenoproteins
Bellinger et al point out that the synthesis of selenoproteins requires a common set of cofactors and adequate dietary selenium intake [Bellinger 2009]. The synthesis of the selenoproteins costs the human body a considerable amount of energy, which suggests just how important the selenoproteins are to the optimal functioning of the cells.
Functions of selenoproteins
The functions of many of the above-listed selenoproteins are not known or are not yet completely known. Some/many, certainly, have a function in cellular antioxidant activity. Rayman lists the glutathione peroxidases, the selenoprotein 15 kDa, the selenoprotein P, and, possibly, the thioredoxin reductases as the selenoproteins having a beneficial role in the prevention of cancer [Rayman 2005].

Illustration: Dr. Margaret P. Rayman, Professor of Nutritional Medicine at the University of Surrey and director of MSc Programme in Nutritional Medicine. Researcher with particular interest in the importance of selenium and iodine to human health.
The known functions of the selenoproteins are diverse. Selenoproteins play important roles in preventing various diseases and conditions such as cancer, cardiovascular disease, thyroid disorders, and neurodegenerative diseases. Bellinger et al list known links between selenoproteins and diseases in Table 1 in their 2009 review article.
Please see the 2009 journal article by Bellinger et al, published in The Biochemical Journal, for an explanation of how changes in the levels of various selenoproteins affect human health. Such an explanation is beyond the scope of this essay. Here, the focus is on the activity of selenoproteins:
- Inactivating free radicals and preventing oxidative damage
- Regulating thyroid hormone function
- Inactivating heavy metals (mercury, lead, cadmium)
- Protecting against cell damage from radiation and environmental toxins
Selenium speciation: all selenium compounds are not the same
Selenium is seldom found in its elemental form. The various chemical forms of the selenium compounds that are found in food and in nutritional supplements are often referred to as selenium species. For example, selenium can be present in either inorganic forms (selenite, selenate, or selenide) or organic forms (selenomethionine, selenocysteine, selenocystine, or other selenoproteins) or both. Particularly in the organic forms of selenium compounds, there are many different species. These selenium species are different in their chemical structure, in their absorption, and in their anti-carcinogenic and other health effects.
Proportions of selenium species not well known
The exact proportions of the various selenium species in foods are not well known [Moesgaard 2001]. Speciation of the various selenium species in food and nutritional supplements and in the bodily fluids and tissues is, accordingly, very important. Speciation provides needed information about the content, quality, and stability of the selenium supplement and about the absorption, transport, storage, and elimination of different forms of selenium in the body.

Illustration: Sven Moesgaard (right), conferring with the Finnish selenium researcher, Dr. Matti Tolonen, was an early leader in the push for speciation of selenium to realize the potential of selenium in disease prevention. Mr. Moesgaard was also very interested in determining the bioavailability of the various species of selenium.
Interpretation of speciation results
Typically, speciation of extractions of various selenium species is done by means of high performance liquid chromatography with inductively-coupled plasma mass spectrometry and gas chromatography with atomic emission detection [Amoako]. The problems encountered in selenium speciation are mostly problems in the interpretation of the results, not in the use of various techniques. In disease prevention, levels of total selenium are less important than the levels of individual selenoproteins [Thomson 1998].
High selenium yeast most effective in disease prevention
The results of randomized controlled trials have shown that selenized yeast supplements are more effective than synthetic selenomethionine supplements in the prevention of cancer [Blot 1993, Clark 1996, Klein 2011]. High selenium yeast supplements contain quantities of both selenomethionine and methylselenocysteine as well as other minor species, depending upon the growth conditions. The task for researchers doing selenium speciation studies is to link the presence of specific species of selenium in the supplements to the presence of the same species in various body tissues and organs and to the concentration levels of specific selenoproteins.
Producing a high selenium yeast supplement
A good high selenium yeast supplement is produced by culturing brewers’ yeast/bakers’ yeast (two strains of the same yeast species, Saccharomyces cerevisiae) in a selenium-rich growth medium and then sterilizing and collecting the micro-organisms that have incorporated the selenium. The yeast cells used to produce the high selenium yeast tablets are subsequently killed by a heating process and are inactive in the tablets themselves.
As many as 30 species in high selenium yeast supplement
A standardized high selenium yeast supplement contains between 54% and 67% natural (not synthetic) organic selenomethionine and as many as thirty other organic species of selenium [Larsen & Moesgaard, 2003]. The proportion of selenomethionine in the high selenium yeast can go as high as 78 or 80 percent. Much depends on the growth conditions. Among the 30 other species are Se- methylselenocysteine, which is thought to play an important role in cancer prevention, and various organic selenium compounds that are thought to be associated with biochemical functions in the human body.
Speciation and bioavailability study
Researchers in Denmark undertook a study of the species speciation and bioavailability of the selenium in the yeast-based intervention agents from three manufacturers [Larsen 2004]:
- Nutrition 21, supplier to the Nutritional Prevention of Cancer (NPC) trial, 1981 – 1996
- Cypress, supplier to the Nutritional Prevention of Cancer (NPC) trial, 1997 – 1999
- Pharma Nord, supplier to the Prevention of Cancer by Intervention by Selenium (PRECISE) trial, 1999 – 2000
The study data yielded the following results:
- The high selenium yeast tablets used in the Nutritional Prevention of Cancer (NPC) trial (described below in the section about selenium supplementation and cancer) contained four predominant species: l-selenomethionine and three other unidentified selenium compounds.
- The SelenoPrecise® high selenium yeast selenium tablets used in the Prevention of Cancer by Intervention with Selenium (PRECISE) trial (also described in the section below) contained more l-selenomethionine, between 54% and 60%, and fewer unidentified selenium compounds.
- The participants in the UK PRECISE pilot trial who took 200 micrograms of the high selenium yeast showed a significantly higher plasma selenium concentration and a higher plasma selenium increase from baseline than did participants taking the same daily dosage in the NPC Trial. The researchers suggested that differences in plasma selenium levels may be explained by differences in the intake, species variation, or bioavailability of the selenium in the different yeast-based formulations.
- The whole blood selenium concentrations in participants in the Danish PRECISE Pilot Trial were significantly higher than the corresponding levels achieved with a synthetic L-selenomethionine preparation used in a comparable group of Danish participants. Both groups, the group taking the high selenium yeast preparation and the group taking the synthetic L-selenomethionine preparation, were treated with 300 micrograms of selenium per day [Larsen & Moesgaard 2004]. L-selenomethionine is the substance that was used in the Selenium and Vitamin E Cancer Prevention Trial (SELECT) (described in the cancer section below).
Speciation of the selenium in selenium supplements in conjunction with clinical trials of specific selenium supplements is important in helping to answer two questions:
- Which species of selenium in the high selenium yeast supplements help to meet basic nutritional needs, e.g. supporting the formation of seleno-enzymes?
- Which species of selenium in the high selenium yeast supplements help to prevent the development of cancer and cardiovascular disease and neurodegenerative diseases?
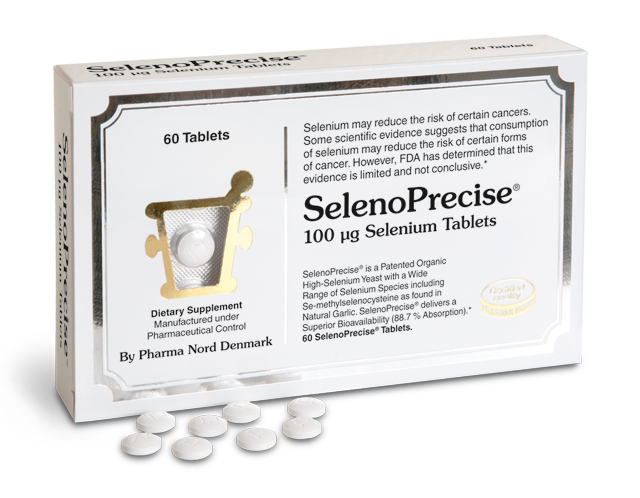
Illustration: The patented Danish high selenium yeast preparation used in the Prevention of Cancer by Intervention with Selenium (PRECISE) pilot studies contains an organic selenomethionine and at least 30 other organic selenium compounds. The many different selenium compounds in SelenoPrecise® serve many different functions in the body.
Selenium: The Need for Supplementation
Lack of adequate quantities of selenium in the soil in various regions of the world can result in low dietary intakes of selenium. The following regions are known to have low quantities of selenium in the soil:
- Northern Europe and the United Kingdom
- New Zealand
- parts of the United States
- parts of China and Russia
- parts of Africa
Northern Europe and Scandinavia are in one of the regions of the world that have the lowest levels of selenium in the agricultural land. In the same way that glacial erosion carved out the deep fjords in Norway, the movement of the glaciers pushed the selenium containing topsoil in Northern Europe farther and farther to the south with the result that the remain soil contains very little selenium. Over the centuries that followed, then, the situation was worsened by pollution and acid rain and intensive farming of the soil [Tolonen 1990].
The consequence of the loss of selenium from the soil in Scandinavia and Northern Europe, as in selenium-poor regions of the United States, was a lack of adequate selenium in agricultural crops, in livestock, and in the people themselves. There were and still are many people with serum selenium status below 85 micrograms per liter, and low levels of selenium status are known to be associated with increased risk of cancer and cardiovascular disease and thyroid disorders [Tolonen 1990].
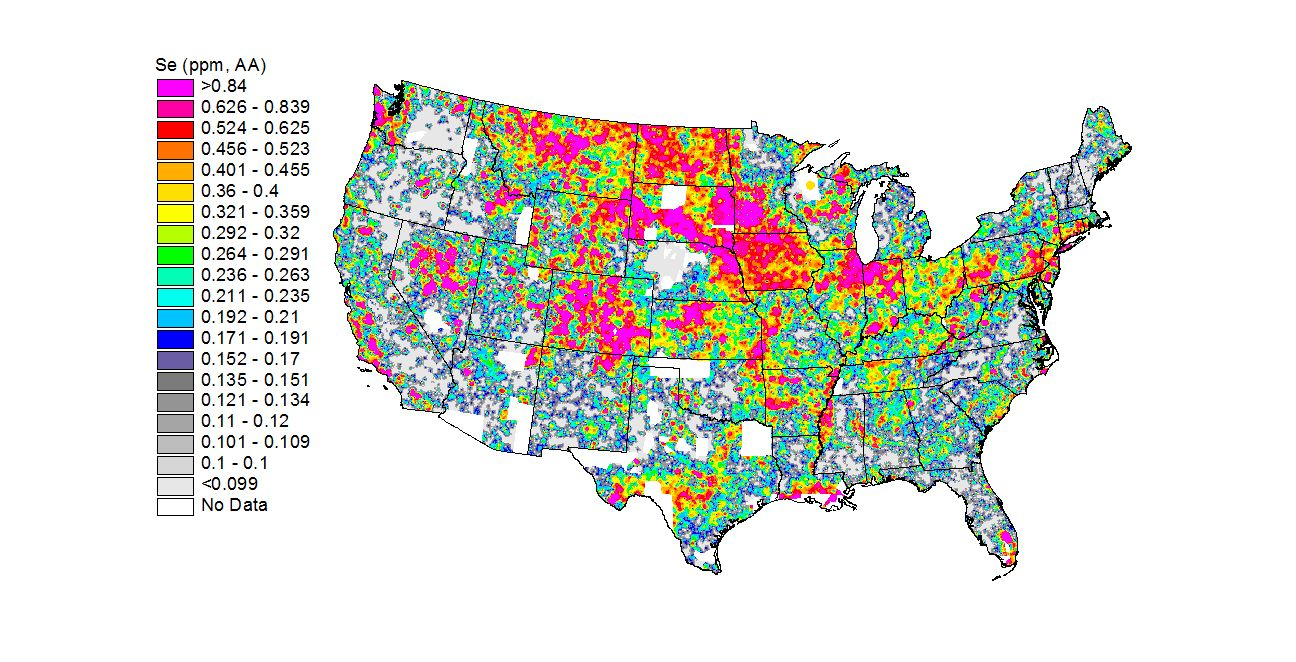
Illustration: Selenium in the US soil in the United States, the states in the Northwest, the Southeast, and the Great Lakes regions tend to have selenium-poor soil. (http://mrdata.usgs.gov/geochem/map/image/lower48/se_aa.jpg)
Deficiency conditions in China
Early reports of selenium deficiency in humans came from China in the 1960s and 1970s: there were reports of Keshan disease, a heart muscle disease, in selenium-poor regions of China and reports of Kashin-Beck disease, a bone disease, in selenium-poor regions of China and Siberia and North Korea [Tolonen 1990].

Illustration: Chinese boy standing on the Great Wall holding a box of Bio-Selenium + Zinc. 1986.
The linking of selenium deficiency to disease conditions in China, Korea, and Siberia provided stimulation for research into the health effects of selenium supplementation and into the role of selenium in the selenium containing antioxidant enzymes [Yang 1988].
As early as 1965, researchers at Xian University in China began experimenting with selenium supplementation as a way to treat victims of Keshan disease, a heart muscle disease that affects young children and women in particular [Tolonen 1990]. Keshan disease in its acute form is characterized by a diminished ability of the heart to pump blood because the left ventricle has become enlarged and weakened.

Illustration: Aware of the need for selenium supplementation in northern Europe, Eli Wallin (far right) and Sven Moesgaard (second from right) had begun producing selenium supplements in 1984. Because there was a much greater knowledge base about selenium and human health in China at that time, Wallin and Moesgaard undertook a study trip to China in 1986. They are pictured here with Professor Tan Jianan, the head of the Department of Chemical Geography at the Chinese Academy of Sciences (on the left) and Finnish researcher Dr. Matti Tolonen (second from left).
Supplementation with selenium was shown to prevent the occurrence of the disease in Keshan province, but it could not reverse the damage to the heart muscle in individuals already afflicted with the disease. Supplementation with selenium has led to a virtual extinction of Keshan disease. Subsequent studies have shown reductions in glutathione peroxidase (GPx) enzyme activity to be prevalent in Keshan disease patients [Lei 2009].
Somewhat later, doctors in parts of China, Tibet, Korea, and Siberia found another disease that was associated with low selenium intakes: Kashin-Beck disease, a disease causing degeneration of the cartilage in the joints. One hypothesized cause of the disease is the lowered antioxidant protection from selenium-containing glutathione peroxidase (GPx) enzymes. A meta-analysis of clinical trials has shown that selenium supplementation in the affected regions is positively associated with treatment of the bone lesions caused by the disease [Zou 2009].

Illustration: Together with Dr. Matti Tolonen, Wallin and Moesgaard made a point of consulting with the leading Chinese researcher in the area of selenium and cancer, Dr. Luo Xianmao of the Cancer Institute of the Chinese Academy of Medical Sciences, pictured here (right) with Dr. Tolonen. Among the topics that they discussed: absorption of various forms of selenium, bioavailability of selenium to Chinese residents in low-selenium regions, importance of selenium for cancer prevention, ramifications of the link between selenium deficiency and Keshan disease and Kashin-Beck disease, and incidence of lung cancer in selenium-poor regions of China.
1912: Handwritten letter dated May 6, 1912, reporting the use of selenium by the French doctors Lancien and Thiroloix to treat a case of cancer of the tongue

Illustration: This letter written by John Gerard Letray of Chicago, Illinois, and dated May 6, 1912, reported the successful use of selenium injections to treat a cancer patient as reported in the French journal La Province Medicale on April 13, 1912.
In Bordeaux, France, the doctors Lancien and Thiroloix treated a 39-year-old patient with cancer of the tongue with selenium. Every eight days, starting on December 19, 1911, and continuing until February 16, 1912, the doctors injected a colloidal selenium solution intravenously. The patient reacted to the injections with alternating fever and chills for about seven hours but then had no adverse effects in the period between the injections.
By January 25, 1912, it was possible to puncture the tumor and draw off fluid. The puncturing and drawing off of fluid continued until mid-February by which time the tumor on the patient’s tongue had disappeared. The doctors noticed no toxic effects from the selenium injections.
Lancien and Thiroloix submitted a report of this successful treatment of cancer with selenium to the Medical Society of the Hospitals of Paris. On March 11, 1912, at a meeting of the society, a Dr. Netter reported on the treatment of a 61-year- old patient suffering from rectal cancer. Weekly injections of the colloidal selenium had improved the general condition of the patient; it was still too early to know the final effect of the injections on the tumor. There are several interesting aspects to this early report of the apparently successful treatment of cancer with selenium:
- The colloidal selenium solution was produced with the application of electricity.
- The colloidal selenium solution was injected intravenously.
- The patient’s reaction to the intravenous injection was relatively mild.
- There were no reported toxic effects of the intravenous injections of selenium.
- How the doctors Lancien and Thiroloix decided to space out the injections to eight days is not known.
- Why the practice of treating cancer tumors with the electro-colloidal selenium did not become more common is not known.
1915: Early report in JAMA of the use of selenium for chemoprevention of cancer
As early as 1915, there were reports in the Journal of the American Medical Association about the possible use of selenium to prevent and shrink cancer tumors. Richard Weil, M.D., of the Cancer Research Service of the General Memorial Hospital in New York was one of the first medical doctors to report on the use of colloidal solutions containing selenium compounds to treat malignant tumors. His report appeared in the Journal of the American Medical Association on April 17, 1915.

Illustration: Dr. Weil attributed the availability of proprietary selenium solutions to the influence of the results of earlier animal studies done by the German scientist Dr. August von Wasserman. Wasserman concluded from his studies that selenium acts to prevent the growth of tumors and to shrink tumors. Wasserman was not satisfied only to inject selenium into local tumors. He wanted to reach tumors that had metastasized to the lymph system and to other organs. For this purpose, Wasserman developed a solution that could be injected into the blood circulation and thus reach all parts of the body. Unfortunately, not much was done with selenium for another 40 years in the field of cancer prevention and treatment.
1935: Mention in the British Medical Journal of success in treating breast cancer patients with selenium
In 1935, Dr. J. P. Lockhart-Mummery published a long paper entitled “Modern Views on the Cancer Problem” in the British Medical Journal. Under the heading “Modern Developments in Treatment,” Dr. Lockhart-Mummery mentioned that certain compounds of selenium had been used successfully to treat cancer [Lockhart-Mummery 1935]. In a follow-up letter to the journal, Dr. F. Hernaman-Johnson made the following points with respect to the treatment of breast cancer tumors with selenium:
- Selenium treatment, in conjunction with x-ray treatment or surgery or both, had been shown to be of some value in treating established cancer. Dr. Hernaman-Johnson urged the testing of selenium for prophylactic purposes.
- In nearly 20 years, up to 1935, of experimental and clinical use of selenium to treat cancer tumors, there was no record of toxic effects of using selenium “in medicinal doses” [Hernaman-Johnson 1935].
- The use of selenium and x-ray treatment together with surgery had resulted in a doubling of the number of five-year survivals in breast cancer cases [Hernaman-Johnson 1935].
1949: Clayton and Bauman report that tumor incidence was decreased by selenium supplementation
Clayton and Bauman tested various substances to see which substances would serve to prevent the induced growth of tumors in the livers of laboratory rats. They reported that tumor incidence was reduced when they supplemented the laboratory rats’ diet with 5 parts per million of sodium selenite, an inorganic form of selenium [Clayton 1949].
1957: Schwarz demonstrates the need for and the efficacy of selenium supplementation
The German-born biochemist Klaus Schwarz was working at the National Institutes of Health in Bethesda, Maryland. In 1957, he published a paper showing that laboratory rats fed a torula yeast diet deficient in selenium developed problems with liver necrosis (death of cells because of disease). When Schwarz switched the rats to a selenium-sufficient baker’s yeast diet, the liver problems disappeared.
The publication of Schwarz’ paper was a turning point in the history of selenium supplementation. Until then, selenium had been regarded with suspicion because, both in animals and humans, high intakes could be toxic. Schwarz’ work made it clear for the first time that selenium in appropriate amounts is essential for optimal health [Tolonen 1990].
Schwarz’ study set in motion much research into the role of selenium in large animal diets in the United States. In the span of the next ten years, many useful studies revealed selenium deficiency to play a role in otherwise unexplained diseases in farm animals. And Schwarz’ work stimulated researchers to investigate the role of selenium in human health and disease. Schwarz is the unsung hero of selenium research [Tolonen 1990].
From 1957 to 1998, then, more than 100 experiments with small animals were carried out to test the relationship of tumor incidence to selenium status (Combs & Gray, 1998).
1969: Shamberger and Frost report anti-carcinogenic effects of selenium
The researchers Shamberger and Frost suspected that selenium availability has an impact on the incidence of cancer. They reasoned that selenium-rich and selenium-adequate regions should have lower rates of cancer than selenium-poor regions. Their analysis of available demographic data revealed a significant inverse relationship between selenium status and incidence of cancer [Shamberger 1969]. The upshot of Shamberger and Frost’s studies was that selenium began to be perceived as a potentially anti-carcinogenic substance.
Much later, in 1999, James Oldfield published a Selenium World Atlas (updated 2002), an 83-page volume detailing the availability or scarcity of selenium in soils, in agriculture, in animal nutrition, and in human nutrition. The selenium world atlas proved especially useful for the charting of areas of selenium deficiency that need selenium supplementation.
1973: Rotruck discovers that selenium is a component of the selenoprotein enzyme glutathione peroxidase
In 1973, Rotruck and his colleagues at the University of Wisconsin published study results that showed that selenium, as a component of the antioxidant enzyme glutathione peroxidase, is essential for human health, and plays a biochemical role in the prevention of oxidative damage. With Rotruck’s work, one of the mechanisms by which selenium affects human health became apparent [Rotruck 1973].
Glutathione peroxidase (abbreviated GPx) is the name given to a family of enzymes with antioxidant properties. At least eight different GPx enzymes have been identified. At least five of the GPx enzymes are antioxidant enzymes [Pillai 2014]. The GPx enzymes protect the body’s cells and tissues against oxidative damage. They work by reducing lipid hydroperoxides to alcohols and by reducing free hydrogen peroxide to water.
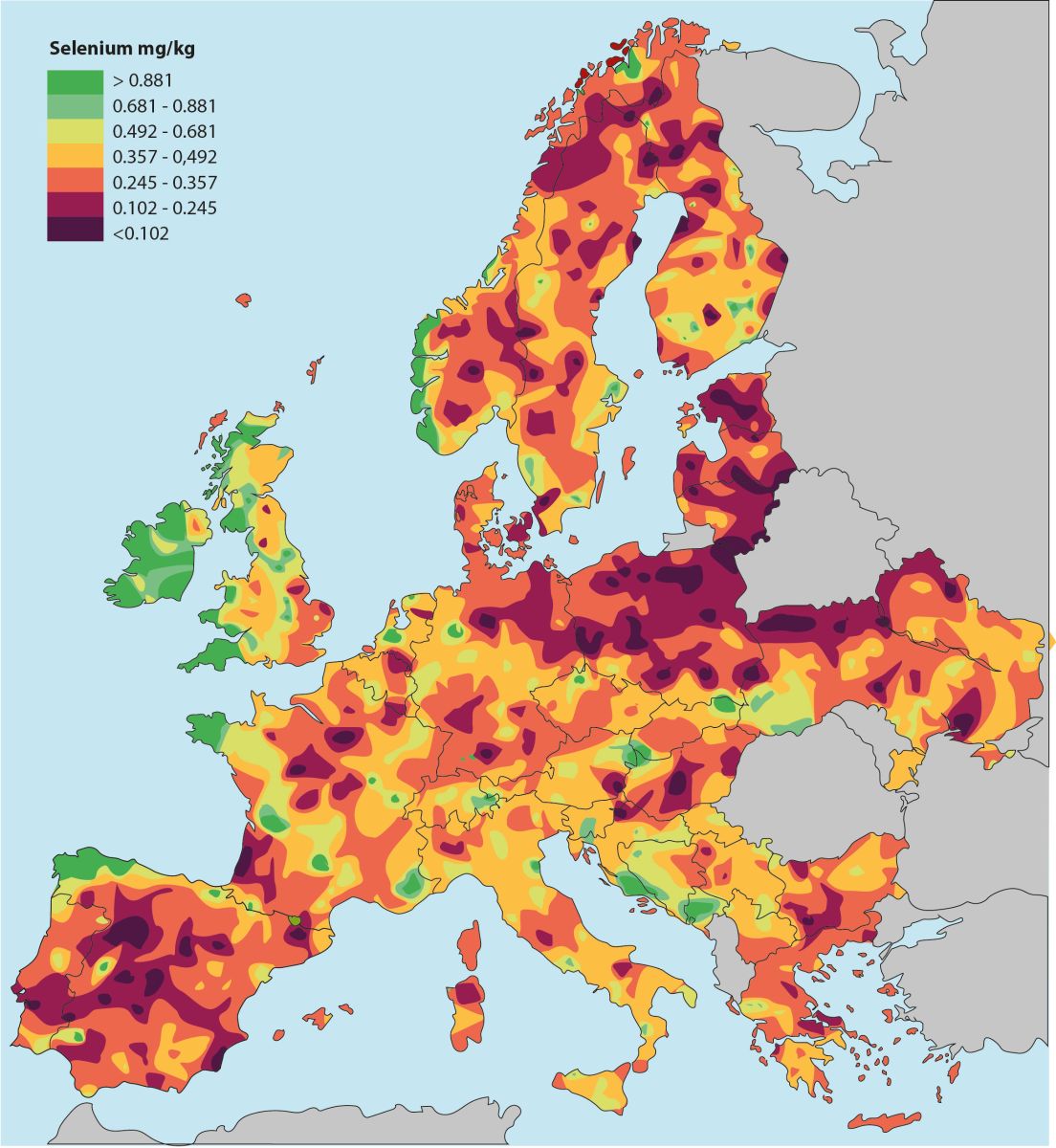
Illustration: The Earth's selenium content is unevenly distributed. Europe has far larger areas than the United States with selenium-poor farmland, but only Finland has taken the consequences of this and has added selenium to fertilizers. However, a group of researchers writes that in order to utilize the Earth's relatively sparse amount of selenium more optimally, selenium should instead be given in the form of supplementation for animals and humans in selenium-poor areas [Haug A, 2007].
1977: Schrauzer does a world-wide analysis of blood-bank data
Dr. Gerhard Schrauzer analyzed the data from the blood banks of 17 countries in the world. He found that the regions with low levels of selenium in the diet were the same regions with higher levels of leukemia and breast cancer, colorectal cancer, lung cancer, ovarian cancer, and prostate cancer. Regions in Europe and the United States that had lower soil selenium levels had from 2 to 5 times greater breast cancer mortality than did regions in Asia and Latin America with higher soil selenium levels [Schrauzer 1977].
1978: Schrauzer reports 70% reduction in breast cancer incidence with selenium supplementation
At the University of California in San Diego, Dr. Gerhard N. Schrauzer experimented with selenium and breast cancer in laboratory mice. He added 2 parts per million of sodium selenite to the drinking water of the laboratory mice with an in-bred cancer-causing virus. After 15 months, 82% of the mice in the control group, having had no selenium added to their drinking water, developed mammary tumors. In the treatment group, only 10% of the mice developed tumors. Furthermore, supplementation with selenium at this level did not have any noticeable adverse effects on the mice’s weight-gain and survival [Schrauzer 1978].

Illustration: German-born and educated Dr. Gerhard N. Schrauzer was one of the pioneers in the selenium and cancer research. Dr. Schrauzer was a long-time professor at the University of California, the director of the Biological Trace Element Research Institute, and the editor-in-chief of the journal Biological Trace Element Research. Internationally, Dr. Schrauzer was recognized as a pioneer in the study of the biological functions of selenium, in particular selenium’s cancer-protective properties. (Picture from the journal Cancer Research dated December 1, 1989)
Selenium: intakes, recommendations, and upper limits
On one level, it would not make sense to list average daily dietary intakes of selenium for individuals because the dietary intakes vary considerably from region to region and from individual diet to individual diet. However, both Rayman [2002] and [Schrauzer [2009} have suggested a minimum intake of 100 micrograms per day.
Recommended dietary allowance
The recommended dietary allowance in the United States for males and females over the age of 14 years is 55 micrograms per day. The recommended dietary allowances seem to be predicated on the saturation of glutathione peroxidase-3 activity in plasma. However, saturation of the glutathione peroxidase-3 in plasma is known to occur at selenium intake levels well below the intake levels needed to saturate the glutathione peroxidase-1 in red blood cells and the glutathione peroxidase-1 in platelets [Bügel & Moesgaard 2008].
In 2011 researchers Joyce McCann and Bruce Ames note that a plasma selenium status at 100 micrograms per liter - based on the maximum activity of glutathione peroxidase - may be insufficient, as selenoprotein P (SePP) has been found to be even more sensitive to selenium deficiency [McCann 2011].
Furthermore, a daily intake of 55 micrograms per day is too low to raise the individual’s selenium status to a cancer-fighting level. Gerald F. Combs, Jr., of Cornell University, has suggested that selenium intakes approximately twice the level of the 55 micrograms per day or more are needed if selenium supplementation is to do more than meet basic biochemical functions [Combs 2001].
Rayman has also found the recommended dietary allowance to be too low. Adequate selenium levels are needed for the optimal functioning of the immune system, and selenium’s anti-carcinogenic effects may depend upon greater than recommended daily intakes. In an article in The Lancet, Rayman suggested minimum daily intake for adults in the range of 80 – 100 micrograms of selenium per day [Rayman 2000].
Tolerable upper intake level
The tolerable upper intake level for selenium in American males and females aged 14 years or older is set at 400 micrograms per day.
Safe intake levels
It can be seen that there is a relatively narrow window for selenium supplementation. Several clinical trials have tested the safety and efficacy of supplementation with 100, 200, or 300 micrograms of selenium daily [Rayman 2011]. The use of high selenium yeast supplements is considered safe; in over three decades of world-wide use as a supplement, there have been no reports of selenium poisoning caused by errors in formulation or dosage [Schrauzer 2006]. In the text below, we will discuss the formulations and the dosages used in the various clinical trials.
Selenium Status
Selenium status varies considerably from region to region and from individual to individual just as selenium intakes do. Moreover, there is some disagreement among researchers as to the best method of measuring selenium status. Measurements in plasma or serum are common. In some studies, researchers use measurement of selenium content in the hair or the toenails.
The significance of the differences of measurements of selenium status in different components of the body needs more research. One example will suffice to illustrate this need: Babaknejad et al did a systematic review of the literature relating selenium status and the incidence of breast cancer. They found that a significant correlation between selenium status and breast cancer could be seen in the studies in which the researchers had measured selenium levels in serum. In the studies in which the researchers had measured selenium levels in toenails, on the other hand, the correlation between selenium status and the incidence of breast cancer was not significant [Babaknejad 2014].
Basically, the commonly accepted cutoff point for selenium deficiency in humans is selenium status in the range of 70 to 85 micrograms per liter (between 0.9 and 1.0 micromoles per liter) [Campa 2012].
Selenium concentrations somewhere in the range of 80 – 100 micrograms per liter of blood are thought to be the minimum status adequate for the bio-synthesis of selenoproteins [National Institutes of Health 2016].
However, there are selenium researchers who think that selenium concentrations of 200 – 250 micrograms per liter are necessary for effective cancer prevention activities [Schrauzer 2009].
The concentration of specific selenoproteins in plasma or serum, e.g. selenoprotein P or glutathione peroxidase, is sometimes measured and then associated with the presence or absence of a disease condition or a bio-marker for a disease condition. In other studies, the level of selenoprotein activity is measured and is used as a biomarker for selenium status. Selenoprotein P is a useful biomarker of status in populations with relatively low selenium intakes because it responds to different dietary forms of selenium.
Hurst reported the results of a randomized, double- blind, placebo-controlled study in which the study participants consumed 50, 100, or 200 micrograms of SelenoPrecise® high selenium yeast tablets or 50 micrograms of selenium of selenium-enriched onions daily. There was a dose- response effect of the supplementation [Hurst 2010].
At baseline, the Hurst study participants – healthy British men and women aged 50 – 64 years – had a plasma selenium status of 95.7 micrograms per liter on average. After ten weeks of supplementation, the plasma selenium values reached the following levels:
118.3 micrograms/liter with 50 micrograms of a SelenoPrecise® supplement daily
152.0 micrograms/liter with 50 micrograms of a SelenoPrecise® supplement daily
177.4 micrograms/liter with 50 micrograms of a SelenoPrecise® supplement daily
Selenium: absorption, transport, storage, and elimination
Danish researchers conducted a single-dose study of the absorption, excretion, and retention of the selenium in a single SelenoPrecise® dose. They gave 12 healthy Danish males -- who had taken 300 micrograms of SelenoPrecise® per day for 10 weeks -- a onetime 327-microgram 77Se isotope preparation (99.3% pure 77Se).
The absorption from a single dose of the 77Se isotope was 88.7% plus/minus 3.9%.
- The retention was 74% plus or minus 6%.
- The average elimination of selenium from the single dose in urine was 47.4 micrograms plus or minus 14.8 micrograms.
- The average excretion in feces was 37.1 micrograms plus or minus 12.7 micrograms.
- The average maximum concentration level for the 77Se selenium isotope in plasma was 9.8 micrograms per liter plus or minus 1.5 micrograms per liter, peaking approximately 8 – 9 hours after ingestion [Bügel & Moesgaard 2008].
Selenium: antioxidant protection against oxidative injury
Selenium is a vital component of various antioxidant enzymes, in particular the glutathione peroxidase enzymes and the thioredoxin reductase enzymes that are selenocysteine-dependent and that protect the cells against oxidative injury. [Venardos 2004]
What is oxidative damage?
Oxidative injury, oxidative stress, and oxidative damage are the various names used to describe the harmful effects of an imbalance in the body between the chemically active free radicals and the antioxidants that are needed to neutralize the free radicals. The most common harmful free radicals are the peroxide, superoxide, hydroxyl radical, and singlet oxygen molecules.
What are free radicals?
The harmful free radicals are generated naturally as byproducts of the normal metabolism of oxygen and by environmental influences such as exposure to ionizing radiation and toxic chemicals. In moderate quantities, the free radicals, called reactive oxygen species, play a necessary role in cell signaling and cell homeostasis.
But, in times of stress, the quantities of free radicals engaging in chain reactions in the body reach harmful levels, and it is the resultant imbalance between the numbers of free radicals and the numbers of neutralizing antioxidants that is the cause of damage to cell structures and to cell DNA.
The damage to cells caused by free radicals has important implications for the development of cancer, the development of cardiovascular disease, and various neurodegenerative diseases [Uttara 2009].
Selenium containing antioxidant enzymes
Selenium serves as a co-factor in the antioxidant enzyme glutathione peroxidase and other selenium containing antioxidant enzymes that are important for the neutralization of harmful free radicals. Cancer prevention and protection against heart disease may come, in part, from selenium’s role in antioxidant activity and, in part, from protection of the cells in other ways.
Selenium: beneficial health effects
In summary, at the time of the writing of this history (winter 2016), it is thought that the primary beneficial health effects of selenium supplementation result from the following activities:
- Detoxification of heavy metals
- Neutralization of free radicals
- Prevention of cancer
- Prevention of the mutation of DNA
- Reduction of the risk of cardiovascular death
- Regulation of thyroid hormone metabolism
- Slowing of the progression from HIV to AIDS
- Treatment of Alzheimer’s disease
Selenium: Chronological history of supplementation in human health:
History of selenium supplementation by topic and by date
At this point in the history of selenium supplementation, it is necessary to present the available research results first by topic and then by date. Research results for the following aspects of human health will be presented:
- Cancer
- Cardiovascular disease
- Thyroid hormone regulation
- Defense against heavy metals
- Neurodegenerative diseases
- HIV/AIDS
- Oxidative Stress
- Diabetes
- Pregnancy
- Elderly
- Smokers
Selenium and Cancer Intervention Studies
1993: Blot reports the results of the Linxian study
The people living in the Linxian County in China had some of the world’s highest rates of cancer morbidity, and they had consistently low intakes of several micronutrients. Blot and a team of researchers began, in 1985, to enroll people aged 40 – 69 years living in four Linxian municipalities. Altogether, they enrolled 29,584 study participants. They randomly assigned the enrollees to one of four treatment groups:
- Group A: taking zinc and retinol (Vitamin A1)
- Group B: taking the B vitamins riboflavin and niacin
- Group C: taking Vitamin C and molybdenum
- Group D: taking selenium, Vitamin E, and beta-carotene
The supplementation regimens in groups A, B, and C were not associated with any significant reductions in all-cause mortality. Supplementation with 50 micrograms of selenium (in a selenium yeast formulation), 30 milligrams of vitamin E, and 15 milligrams of beta-carotene showed several statistically significant reductions in mortality rates:
- 9% reduction in total mortality
- 13% reduction in cancer mortality
- 21% reduction in gastric cancer mortality
- 20% reduction in mortality from other cancers
These research results were the first results from a large nutritional intervention study that suggested a link between supplementation with a selenium yeast preparation and a reduction in the number of cancer deaths.
The results of a nested study drawing on 1103 participants in the Linxian study, reported in 2004, showed significant inverse associations between baseline serum selenium and death from esophageal squamous cell carcinoma and from gastric cardia cancer. There was also a trend toward an inverse association for death from heart disease but no similar trend for death from stroke [Wei 2004].
1994: Beginning of the French Su.Vi.Max study
Inspired perhaps by the Linxian study results, French researchers began in 1994 to recruit participants for the Supplémentation en Vitamines et Minéraux Antioxydants (Su.Vi. Max) study, a randomized, double-blind, placebo-controlled primary prevention trial. Altogether, the researchers enrolled 13,017 French adults (7876 women aged 35-60 years and 5141 men aged 45-60 years) from the general population, without regard for disease risk factors such as smoking or occupational exposure to toxins [Hercberg 2004].
All participants took a single daily verum capsule or corresponding placebo capsule. The verum capsule contained the following substances:
- 100 micrograms of selenium (as high selenium yeast)
- 120 milligrams of ascorbic acid
- 30 milligrams of vitamin E
- 20 milligrams of zinc
- 6 milligrams of beta carotene
The median follow-up period for the Su.Vi. Max study was 7.5 years. The study data showed that the 7.5 years of low-dose antioxidant supplementation (including selenium) lowered total cancer incidence and all-cause mortality in men but not in women. Moreover, antioxidant supplementation was positively associated with a greater healthy aging probability among male participants but not among female participants [Assman 2015].
The French researchers made the following comments on the results of the Su.Vi.Max study:
- The selenium dosage used in the Su.Vi.Max study was low. It was a nutritional dosage rather than a pharmaceutical dosage.
- The participants in the Linxian study had had lower baseline antioxidant status than the participants in the Su.Vi.Max study.
- The participants in the Su.Vi.Max study were recruited without any exclusion of individuals with specific disease risk factors.
- The antioxidant supplementation may have been more effective in men than in women because the women had had higher baseline vitamin C and beta-carotene status. Furthermore, there may have been hormonal differences at play in the differing results.
- The study data showed that baseline antioxidant status was related to the risk of cancer in men but not to the risk of cancer in women. Consequently, differences in baseline antioxidant levels cannot entirely explain the sex differences in the effect of antioxidant supplementation on cancer risk [Galan 2005].
1995: Prasad presents evidence of Indian cancer chemoprevention
Prasad et al randomly assigned 298 Indian patients with precancerous lesions in their mouths to an intervention group or a placebo group. For one year, half of the patients received a daily combination of four micronutrients: vitamin A, riboflavin, zinc, and selenium. The selenium was in the form of a selenium enriched yeast with a dosage of 100 micrograms daily for the first six months and 50 micrograms the last six months. The other half of the patients received matching placebos [Prasad 1995].
The micronutrient supplementation significantly reduced the frequency of micronuclei and DNA adducts. There was no reduction in the placebo group. The presence of micronuclei is usually a bio-marker for increased DNA damage or mutation. DNA adducts are segments of DNA that are bound to carcinogenic molecules.
1996: Clark releases the results of the four-year Nutritional Prevention of Cancer (NPC) study
The big breakthrough in selenium and cancer research came when Clark and his fellow researchers reported the results of the NPC study in JAMA (the Journal of the American Medical Association). The researchers enrolled 1,312 non-melanoma skin cancer patients in a multi-center, randomized, double-blind, placebo-controlled study and followed the study participants for a total of 8,271 person-years [Clark 1996].
There were no statistically significant reductions in the study’s primary endpoints, which were the incidence of basal cell carcinoma and squamous cell carcinoma. However, the effects of daily supplementation with 200 micrograms of a high selenium yeast preparation over a four-year period were thought-provoking:
- 37% lower total cancer incidence
- 46% lower lung cancer incidence
- 58% lower colorectal cancer incidence
- 63% lower prostate cancer incidence
- 50% lower cancer mortality
The study participants were predominantly American men living in selenium-poor regions in the southeastern United States. They were treated with a selenium supplement for an average of 4.5 years. Because the sample was so heavily male – approximately 75% –, there were no meaningful breast cancer statistics.
A 2002 re-analysis of the NPC study data for lung cancer revealed that selenium supplement did not significantly decrease the risk of lung cancer in the entire 1,312-person sample but did significantly decrease lung cancer risk in the tertile of participants with the lowest baseline selenium concentrations [Reid 2002].
A 2008 analysis of the study data from a sub-set of the NPC clinical trial in which 424 participants in the Macon, GA, area had been randomly assigned to take 400 micrograms (as opposed to the 200 micrograms in the rest of the NPC study) or matched placebo showed no effect of the 400-microgram per day selenium supplementation on total cancer incidence [Reid 2008].

Illustration: Dr. Larry Clark (left), lead researcher on the Nutritional Prevention of Cancer study, and Sven Moesgaard discussed various formulations of selenium supplements and the effect on cancer prevention in Tucson, Arizona.
1997: Yu reports a protective role for selenium against hepatitis B virus and primary liver cancer
Yu et al reported the results of intervention studies of the effect of selenium
supplementation on hepatitis B virus (HBV) infection and primary liver cancer in Qidong County in China. Supplementation in the form of selenized table salt showed a 35% reduction in primary liver cancer as compared to the non- supplemented individuals sampled. The incidence of primary liver cancer began to rise in the treated group following the withdrawal of the selenized supplement.
In a related intervention study, Yu et al followed 226 hepatitis B patients for four years. None of the 113 patients randomized to the treatment group – 200 micrograms of high selenium yeast daily – were diagnosed with primary liver cancer during the study. Seven of the 113 patients randomized to the control group – no selenium supplement – were diagnosed with primary liver cancer. After the selenium treatment was discontinued, primary liver cancer rates in the former treatment group began to develop similar to the rates in the control group, indicating that continuous selenium supplementation may be necessary for optimal chemopreventive effect [Yu 1997].
1998: Clark reports effect of selenium on prostate cancer in the Nutritional Prevention of Cancer trial
Clark et al analyzed the data from 974 of the male participants in the Nutritional Prevention of Cancer trial and reported the results in the British Journal of Urology. The 974 men had a history of either basal cell carcinoma or squamous cell carcinoma and were randomized to receive either 200 micrograms of high selenium yeast or corresponding placebo daily. They were treated for an average of 4.5 years and followed for an average of 6.5 years.
The patients in the selenium treatment group had a 63% reduction in the incidence of prostate cancer compared to the patients in the placebo group. When the analysis was restricted to the data from the 843 patients who had had PSA levels less than or equal to 4 nanograms per milliliter at the beginning of the study, then the risk reduction was 74%.
The treatment with 200 micrograms daily of a high selenium yeast preparation resulted in substantial reductions in prostate cancer incidence, total cancer incidence, and total cancer mortality [Clark 1998].
1998-1999: PRECISE (PREvention of Cancer by Intervention with SElenium) study begins in Denmark and in the United Kingdom
The PRECISE (PREvention of Cancer by Intervention with SElenium) trial was originally conceived as the definitive study of the effect of selenium supplementation in the form of high selenium yeast tablets on the risk of cancer in healthy elderly citizens. The PRECISE study was intended to be a randomized, double-blind, placebo-controlled study enrolling 32,000 healthy participants aged 60 – 74 years in centers in Denmark, Sweden, the United Kingdom, and the United States. The participants were to be evenly distributed between male and female [Moesgaard 2001].
At its completion, the PRECISE study was designed to have 90% statistical power to detect a 15% reduction in total cancer incidence and a 32% reduction in prostate cancer incidence. Such results from the PRECISE study would have confirmed the findings of the Nutritional Prevention of Cancer study, the Linxian study, and the Su.Vi.Max study, all of which were studies that had been conducted with high selenium yeast supplements.
The PRECISE trial participants were to receive 100, 200, or 300 micrograms of a high selenium yeast or placebo daily for a period of five years. The Danish company Pharma Nord provided the high selenium yeast tablets (SelenoPrecise®), and the first participants were enrolled in pilot studies in Denmark in November of 1998 and in the United Kingdom in the autumn of 1999.
PRECISE trials not completed because of lack of funding
Unfortunately, the organizers of the PRECISE study were not able to secure the necessary funding for a full-blown 32,000-participant study, and the only research results to date come from pilot studies conducted in Denmark and the United Kingdom.
The outcomes of the PRECISE pilot studies will be reported here even though the publication of the results came years later.
Larsen and Rayman report selenium speciation and bioavailability data from the PRECISE pilot studies in Denmark and the United Kingdom
In 2004, Erik Larsen of the Danish Institute for Food and Veterinary Research and Margaret P. Rayman of the University of Surrey in the UK drew on the results of two PRECISE study samples:
483 participants aged 60-74 years in the UK PRECISE pilot study
496 participants aged 60-74 years in the Danish PRECISE pilot study
The participants in the two samples received SelenoPrecise® in doses of 100, 200, or 300 micrograms per day. In the two pilot studies, six months of supplementation with 100, 200, and 300 micrograms per day increased the plasma and whole blood levels, respectively, as shown in the tables below.
| UK PRECISE Study | |
|---|---|
| Supplementation | Change in plasma levels (mean +/- standard deviation) |
| 100 mcg/day | from 92 +/- 20 ng/ml to 148 +/- 28 |
| 200 mcg/day | from 92 +/- 20 ng/ml to 196 +/- 42 |
| 300 mcg/day | from 92 +/- 20 ng/ml to 233 +/- 54 |
| Denmark PRECISE | Study |
|---|---|
| Supplementation | Change in whole blood levels (mean +/- standard deviation) |
| 100 mcg/day | from 96 +/- 9 ng/ml to 177 +/- 18 |
| 200 mcg/day | from 96 +/- 9 ng/ml to 308 +/- 78 |
| 300 mcg/day | from 96 +/- 9 ng/ml to 441 +/- 132 |
The segment of the UK PRECISE participants who took 200 micrograms of
SelenoPrecise® selenium per day showed a significantly higher plasma selenium level and a higher increase from baseline than the patients who took 200 micrograms per day of a different yeast-based selenium preparation in the Nutritional Prevention of Cancer trial.
Moreover, the selenium concentration obtained in whole blood with the 300 mcg/day of SelenoPrecise® used in the Danish PRECISE Pilot Trial was significantly higher than the whole blood concentration achieved by 300 micrograms per day of a synthetic L-selenomethionine preparation taken by a comparable group of Danes [Larsen 2004].
More results from the PRECISE pilot studies are reported below in the Selenium and Cardiovascular Disease section and in the Selenium and Thyroid Function section.
2000: Li reports on selenium supplementation for primary liver cancer patients
Li et al randomly assigned 2,065 patients to an experimental group that received an inorganic sodium selenite supplement or to a control group that received a placebo tablet every day for three years.
The selenium supplementation group’s blood selenium concentration and glutathione peroxidase activity increased significantly compared with the placebo group. Moreover, the proportion of micronucleus cells in peripheral white blood cells in the patients in the experimental group was significantly lower than in the placebo group. Micronuclei are cells with DNA damage. The incidence of new liver cancers was also significantly lower in the experimental group.
The researchers concluded that the study results show that selenium supplementation has a chemopreventive effect in general populations at high risk of liver cancer [Li 2000].
2001 – 2004: The Selenium and Vitamin E Cancer Prevention Trial (SELECT)
From 2001 to 2004, researchers enrolled 35,533 men from 427 medical treatment sites in the U.S., Canada, and Puerto Rico. Minorities made up 21% of the total enrollment; 15% of the participants were African Americans. The study supplementation ended in 2008.
SELECT was a randomized, placebo-controlled trial of selenium and vitamin E supplementation for the prevention of prostate cancer. The researchers enrolled men who, at the time of enrollment, were 55 years of age or older (50 years for African Americans), had no prostate-cancer diagnosis, and had a PSA equal to or less than 4 nanograms per milliliter. The participants in the study underwent semi-annual clinical exams as well as annual cancer screening with PSA blood tests and digital rectal exams.
The participants were randomly assigned to one of four groups:
- Selenium group: 200 micrograms per day of a synthetic l-selenomethionine preparation
- Vitamin E group: 400 international units per day of an exclusively rac-α-tocopheryl acetate formulation
- Combination selenium and Vitamin E group: dosages as above Placebo group: matched to the selenium and Vitamin E
SELECT trial halted early
The SELECT study researchers’ intention was to follow the participants for a minimum of 7 and a maximum of 12 years. In October, 2008, the SELECT trial was halted ahead of schedule because the primary results indicated that neither selenium nor Vitamin E, alone or in combination, was associated with the prevention of prostate cancer. In 2011, the researchers reported an updated analysis of the study data that showed no effect on prostate cancer for the selenium group and increased risk for the Vitamin E group compared to placebo [Lippman 2009, Klein 2011].
Understanding the SELECT study results
The results of the SELECT study seemed to underscore the fact that different forms of selenium supplements have different effects. Selenium supplementation in the form of a high selenium yeast preparation has been positively associated with risk reduction in various forms of cancer [Blot, 1993, Clark 1996, Hercberg 2010]. The preparation used in the SELECT study was a synthetic selenomethionine preparation. It did not show the same effect. That information is useful.
Moreover, there is evidence that selenium supplementation in tandem with a proper mixture of vitamin E forms might have preventive effects in prostate cancer. Ju et al, writing in the journal Carcinogenesis in 2010, provided evidence that the gamma-tocopherol form of vitamin E, which was not used in the SELECT study, is the form of vitamin E that is most effective in cancer prevention [Ju 2010].
Not only has alpha-tocopherol, the form of vitamin E used in the SELECT study, not been shown to prevent cancer; its exclusive use is thought to reduce the availability and effectiveness of gamma-tocopherol. Helzlsouer et al reported in the Journal of the National Cancer Institute that they observed statistically significant chemopreventive effects of selenium and alpha-tocopherol supplementation only when the study participants’ gamma-tocopherol concentrations were high. [Helzlsouer 2000].
Kristal et al, using selenium status data from toenail selenium concentrations, did a cohort case study and found that the selenomethionine used in the SELECT study did not show a significant reduction of prostate cancer risk in men with low baseline selenium status and showed an increase in high-grade prostate cancer risk in men with high baseline selenium status [Kristal 2014].
Geybels et al, on the other hand, in the prospective Netherlands Cohort Study, showed that higher toenail selenium concentrations were associated with a substantial and significant decreased risk of advanced prostate cancer [Geybels 2013].
Synthetic unbound selenomethionine in the SELECT study
Dr. Gerhard Schrauzer of the University of California, San Diego, has pointed out that selenomethionine is one of the primary selenium species in selenium yeast but is, in the selenium yeast, protein-bound whereas the selenomethionine was free (unbound) in the preparation used in the SELECT trial. Selenomethionine that is protein-bound in selenium yeast preparations may be better protected against oxidation and may be, therefore, more effective against cancer. It may be that the uptake, transport, metabolism, and elimination of free selenomethionine and protein-bound selenomethionine are different [Schrauzer 2009].
Higher baseline selenium status
Dr. Schrauzer also pointed out that the SELECT participants had considerably higher baseline selenium status than did the patients in Clark’s NPC study. Schrauzer thought that, perhaps, the selenium levels of the SELECT placebo group were sufficiently high from the start of the trial as to make improvements in the selenium treatment group unobservable [Schrauzer 2009].
Dr. Schrauzer rejected the idea that the results of the SELECT study suggest that all forms of selenium are ineffective against cancer. Hatfield and Gladyshev, National Cancer Institute, concurred with Schrauzer that the form of the selenium supplement, synthetic l-selenomethionine, and the relatively high baseline selenium status in the participants may have contributed to the unexpected outcome in the SELECT trial [Hatfield 2009].
Margaret P. Rayman and G. F. Combs, Jr. came to similar conclusions [Rayman 2009]. The thinking about variations in the effectiveness of selenium in cancer prevention according to plasma or serum levels stems in great part from work done by Bleys et al at Johns Hopkins University. Bleys measured serum selenium levels in 13,887 adults, a representative sample of adults in the USA [Bleys 2008]. The average serum selenium level was 125.6 nanograms per milliliter (bearing in mind that there is wide range in serum selenium levels in the United States).
Nonlinear association between selenium status and mortality
Bleys’ study showed that the association between serum selenium levels and all-cause mortality and cancer mortality is nonlinear [Bleys 2008]. There is, apparently, an inverse association between selenium and cancer at low selenium status levels (less than 130 nanograms per milliliter) and then modest increase in overall cancer mortality at higher selenium levels (greater than 150 nanograms per milliliter).
2005: Etminan does a meta-analysis of studies relating intake of selenium to the prevention of prostate cancer
Etminan and researchers at the Royal Victoria Hospital in Montreal, Quebec, did a systematic review of the research literature from 1996 to 2005 dealing with the association between the daily intake of selenium and the risk of prostate cancer. They analyzed 16 studies: 11 cohort studies and 5 case-control studies and no randomized, controlled trials. The pooled results of the studies showed a 26% decrease in the risk of prostate cancer associated with higher selenium intakes. Study participants whose daily intakes were increased by selenium supplementation rather than dietary selenium had potentially higher bioavailability for selenium and a potentially more pronounced protective effect of selenium.
The researchers concluded that increased selenium intake may reduce the risk of prostate cancer. Moreover, they pointed out that any protective effect of selenium is dependent upon the actual form of the selenium [Etminan 2005].
2006: Reid studies the association between selenium supplementation and colorectal adenomas using data from the NPC trial
Reid et al did a secondary analysis of the data from the Nutritional Prevention of Cancer trial relating to the incidence of colorectal cancers. The study data showed significant risk reduction in two set of participants:
- NPC trial participants who were in the lowest tertile of baseline selenium status
- NPC trial participants who were current smokers [Reid 2006]
2011: Marshall investigates the use of selenomethionine in patients with premalignant lesions
Marshall et al analyzed the data from a three-year randomized, double-blind, placebo-controlled trial of daily 200 micrograms of selenium as selenomethionine in 699 men with premalignant lesions associated with an increased risk of prostate cancer.
The researchers found a nonsignificantly reduced prostate cancer risk for the selenium treatment in the lowest quartile of baseline plasma selenium level (levels below 106 nanograms per milliliter). In the other three quartiles of selenium status, the selenium supplementation with selenomethionine showed no effect on prostate cancer risk.
The researchers recommended future study of selenium supplementation in selenium-deficient populations. The selenium status in the lowest quartile in this study was not particularly low, and the selenium status in the other three quartiles was above normal [Marshall 2011].
2011: Lee does a meta-analysis of selenium supplementation and cancer prevention
Lee et al analyzed the results of nine randomized controlled trials of the chemopreventive effect of selenium supplementation on cancer. The nine trials accounted for 152,538 total participants, 32,110 of them having received an active antioxidant supplement treatment and 120,428 having received matching placebos.
Selenium supplements alone, not in combination with other antioxidants, were found to have an overall preventive effect on cancer incidence. The relative risk was shown to be 0.76. Study participants taking a selenium supplement had a 24% lesser risk of developing cancer than did study participants taking the matching placebo.
Lee et al concluded that there is possible evidence to recommend the use of selenium supplementation in individuals with low baseline selenium status (defined as less than 125 nanograms per milliliter) and in individuals in populations at high risk for cancer [Lee 2011].
2012: Goossens designs the SELEBLAT selenium and bladder cancer trial in Belgium
Observational studies have consistently shown that selenium concentrations are inversely associated with the risk of bladder cancer. Researchers in Belgium are testing the hypothesis that selenium supplementation may prevent the recurrence of bladder cancer. The Selenium and Bladder Cancer Trial (SELEBLAT study) is a phase lll randomized, double-blind, placebo-controlled chemoprevention study.
The researchers began recruiting patients in September 2009 and are still recruiting patients diagnosed with non-invasive transitional cell carcinoma of the bladder in TURB operations in 15 Belgian hospitals. The recruited patients are given their standard care and are randomly assigned to a SelenoPrecise® selenium yeast (200 micrograms/day) supplementation group or to a matching placebo group for a three-year period [Goossens 2012].
Future reporting on the results of the SELEBLAT study will reveal whether selenium supplementation in the form of high selenium yeast will significantly reduce the recurrence of bladder cancer.
2013: Bonelli reports the results of antioxidant supplementation and long-term reduction of recurrent adenomas of the large bowel in Italy
In this randomized, controlled study, Bonelli et al in Genoa, Italy, enrolled 411 patients who had a clean colon after the removal of polyps. The researchers then did colonoscopy on the patients at the 1-year, 3-year, and 5-year points of the study.
The endpoint of the study was any recurrence of an adenoma (a non-cancerous tumor). The rationale for the study was that patients who have had polyps removed are at greater risk of a recurrence and that reducing the occurrence/recurrence of adenomas should reduce the risk of colorectal cancer [Bonelli 2013].
The active intervention in the Bonelli study consisted of daily supplementation with the Bio-Selenium + Zinc antioxidant preparation:
- 200 micrograms of selenium (as l-selenomethionine)
- 30 milligrams of zinc gluconate
- 2 milligrams of Vitamin A
- 4.4 milligrams of Vitamin B6
- 180 milligrams of Vitamin C
- 30 milligrams of Vitamin E
Patients in the control group took a daily placebo.
The study data have shown a 39% reduction in adenoma recurrence in the selenium treatment group as compared with the placebo group. The risk reduction held for both small tubular adenomas and advanced adenomas. Thus, the study has shown a statistically significant effect of selenium-based antioxidant supplementation on adenoma recurrence in patients considered to be at increased risk of colorectal cancer [Bonelli 2013].
2013: Algotar investigates use of selenium in high risk prostate cancer patients
Algotar et al investigated the effect of supplementation with 200 and 400 micrograms of a high selenium yeast daily in a randomized, double-blind, placebo-controlled clinical trial. The researchers enrolled 699 men at high risk for prostate cancer, defined as patients with prostate specific antigen (PSA) levels greater than 4 nanograms per milliliter or with suspicious digital rectal examinations or with PSA velocity greater than 0.75 nanograms per milliliter per year but with a negative prostate biopsy.
The researchers checked the patients every 6 months for up to 5 years. The selenium supplementation appeared not to have a significant effect on the incidence of prostate cancer in men at high risk.
2014: Vinceti updates the Cochrane review of selenium and cancer studies
Vinceti et al included 55 observational studies and 8 randomized controlled trials in their survey. In the observational studies, they found statistically significant inverse associations between selenium exposure, on the one hand, and cancer incidence and cancer mortality, on the other hand. In the aggregate results from the randomized controlled trials, they could not find the same clear-cut evidence that selenium supplementation reduces the risk of cancer. The participants in the 8 randomized controlled trials were predominantly male; consequently, gender differences could not be evaluated. More and better clinical trials are needed to establish a causal relationship between selenium supplementation and the reduction of the risk of developing cancer.
2014: Richie and El-Bayoumy compare efficacy of selenium-enriched yeast and selenomethionine tablets
Richie and el-Bayoumy of the Pennsylvania State University conducted a direct comparison study of the effects of high selenium yeast tablets and selenomethionine tablets on prostate cancer biomarkers in men.
They performed a 9-month randomized, double blind, placebo-controlled trial in which 69 healthy men were randomly assigned to take either 200 or 285 micrograms per day of a high selenium yeast preparation, 200 micrograms per day of a synthetic selenomethionine preparation, or a placebo tablet. The researchers reported that compliance was high in all groups [Richie 2014].
The primary endpoints of the study were blood levels of selenium-containing compounds and oxidative stress biomarkers. The secondary endpoints of the study were plasma glucose levels and PSA levels.
The data from the study showed that levels of two oxidative stress bio-markers decreased 34% and 28%, respectively, after 9 months in the 285 microgram/day high selenium yeast group. The researchers could see that the decreases were greatest in individuals who had had low baseline plasma levels of selenium (defined as lower than 127 nanograms per milliliter.
There were no significant changes in PSA or blood glucose or glutathione peroxidase levels.
The Richie and El-Bayoumy Penn State study shows that supplementation with a high selenium yeast preparation is positively associated with reductions in biomarkers of oxidative stress in healthy men. Supplementation with a synthetic selenomethionine preparation did not produce similar results. The Penn State researchers concluded that some selenium-containing compounds other than selenomethionine must explain the decrease in oxidative stress [Richie 2014].
2015: Karamali reports beneficial effects in patients with cervical intraepithelial neoplasia
Karamali et al conducted a randomized, double-blind, placebo-controlled study of the effects of a six-month administration of high selenium yeast supplements to women with cervical intraepithelial neoplasia grade 1 (CIN1). The dosage was 200 micrograms per day.
The study results after six months showed the following statistically significant health benefits in the selenium supplementation group as compared with the placebo group:
- Regression of the cervical intraepithelial neoplasia (CIN is the precancerous abnormal growth of squamous cells on the cervix.)
- Decreases in fasting plasma glucose levels and in serum insulin levels
- Increases in insulin sensitivity check index scores
- Decreases in serum triglyceride levels
- Increases in HDL-cholesterol levels
- Decreases in malondialdehyde levels (the presence of malondialdehyde is a biomarker for oxidative stress)
- Increases in plasma total antioxidant capacity and glutathione antioxidant enzyme levels
The researchers concluded that Se supplementation of patients with CIN1 led to its regression and also had beneficial health effects on the patients’ metabolic profiles [Karamali 2015].
2016: Cai does a meta-analysis of selenium and cancer research studies
Cai et al analyzed 69 studies. The results showed that high selenium exposure had a protective effect on cancer risk: high serum or plasma selenium and toenail selenium were positively associated with cancer prevention. However, in the analysis, selenium supplementation did not reach the same clear level of protective effect.
2016: University of Arizona Cancer Center Selenium for Prevention of Adenomatous Colorectal Polyps trial nearing completion
Thompson et al began the Selenium and Celecoxib (Sel/Cel) Trial for colorectal adenoma prevention in November 2001. Originally, the researchers envisioned three study groups: a celecoxib treatment group, a high selenium yeast treatment group, and a placebo control group. However, in December 2004, the celecoxib treatment group was discontinued because of reports of cardiovascular toxicity caused by COX-2 inhibitors. The study continued with just the selenized yeast selenium treatment group and the control group [Thompson 2016].
The randomization of the 1,621 participants was completed in November 2008. Then, researchers added 200 more participants with one or more advanced adenomas
(adenomas = benign tumors associated with increased risk of colorectal cancer). The final cohort of 1,824 participants was completely formed by January 2011.
The gender breakdown was roughly 65% male and 35% female. The average age of the participants was 63 years. The range in ages was from 40 to 80 years. Approximately 9/10 of the participants were Caucasian. 20% of them had three or more adenomas, and 38% had advanced adenomas. The participants in the selenium part of the study received 200 micrograms of high selenium yeast daily or matching placebo for a median period of 33 months.
At the end of the study, data for analysis were available from 689 participants in the placebo group and 685 participants in the high selenium yeast group. The researchers presented the following findings: no statistically significant reduction in the recurrence of adenomas overall, a statistically significant 18% reduction in the recurrence of adenomas in participants who had had advanced adenomas at baseline, and a statistically significant increase of recurrence of multiple adenomas (in those cases when there was a recurrence with multiple adenomas) in men but not in women [Thompson 2016]..
What have we learned from the selenium and cancer clinical trials?
The precise mechanisms by which selenium supplementation inhibits the development of cancer tumors is still (in 2016) not well known. Based on the above review of the results of clinical trials, it seems possible to make three important claims about the chemopreventive mechanisms of selenium:
- Selenium supplementation in the form of high selenium yeast is mostly likely to be effective.
- Selenium supplementation at pharmacological dosages, i.e. doses above the normal nutritional doses, is more likely to be effective: dosages in the range of 200 – 300 micrograms per day. However, the supplemental dosage will need to vary according to the selenium content of the individual’s diet and according to the individual’s baseline selenium status.
- Selenium supplementation of individuals with low selenium status at baseline is more likely to show results in the long term.
We need further investigation of the following mechanisms for cancer prevention by selenium supplementation [Lü 2016]:
- Selenium supplementation’s effect on apoptosis
- Selenium supplementation’s effect on the role of the selenoenzymes
- Selenium supplementation’s effect on carcinogen metabolism
- Selenium supplementation’s effect on the immune system
- Selenium supplementation’s effect on the inhibition of tumor cell growth by the action of selenium metabolites
Selenium and Cardiovascular Disease Intervention Studies
1994: Kuklinski uses selenium and other antioxidants to treat acute heart attack patients
Kuklinski in Rostock, Germany, randomly assigned 61 acute heart attack patients with symptoms of less than 6 hours’ duration upon admission to hospital to one of two groups: an antioxidant supplementation group (n=32) or a placebo control group (n=29).

Illustration: Dr. Bodo Kuklinski was a pioneer in the use of Coenzyme Q10 and selenium to protect heart disease patients from oxidative damage.
The patients in the antioxidant supplementation group received, initially, 500 micrograms of inorganic selenium (in the form of sodium selenite) and then received daily supplements of 100 micrograms of selenium (in the form of l- selenomethionine) plus 100 milligrams of Coenzyme Q10 for a period of one year [Kuklinski 1994].
The patients in the control group (n = 29) received matching placebo preparations. The study data showed that the patients in the two groups were similar in age, sex, and medical treatment.
Dr. Kuklinski used an electrocardiogram to measure electrical impulses in the patients’ hearts. He measured the space between the start of the Q wave and the end of the T wave (called the QT interval). The QT interval gives an estimate of the time that it takes for the heart to pump out blood and then refill with blood. Dr. Kuklinski wanted to see if the patients’ hearts were contracting and refilling within a normal amount of time.
Prolonged QT intervals would be a sign of abnormalities in the heart's electrical waves. None of Dr. Kuklinski’s patients in the selenium and Coenzyme Q10 antioxidant supplementation group showed a prolongation of the QT-interval. In the control group, however, 40% showed a significant prolongation of the QT- interval of more than 440 milliseconds.
In the one-year follow-up period after the patients’ heart attacks, six patients (20%) in the control group died from a second heart attack. One patient in the antioxidant supplementation group died, from a non-cardiac death.
2005: Witte tests the use of a micronutrient combination including selenium on left ventricular volume and ejection fraction
Witte et al investigated the effect of a nine-month multiple micronutrient supplementation regimen on left ventricular (LV) function, levels of pro-inflammatory cytokines, and quality-of-life in elderly patients with chronic heart failure. The patients in the active treatment group received a combination of micronutrients including selenium, Coenzyme Q10, calcium, magnesium, zinc, copper, vitamin A, various B vitamins, vitamin C, vitamin D, and vitamin E in addition to their conventional heart failure medications. The patients in the placebo group received matching capsules and tablets [Witte 2005].
The micronutrient supplementation was significantly positively associated with a reduction in left ventricular volume; there was no change in the placebo group. In the micronutrient supplementation group, the left ventricular ejection fraction increased significantly; in the placebo group, it remained unchanged.
The patients in the micronutrient supplementation group also recorded a significant improvement in their quality of life scores; the patients in the placebo group recorded a slight deterioration in quality of life.
The researchers concluded that long-term multiple micronutrient supplementation can improve left ventricular volume and ejection fraction and quality-of-life scores for elderly patients with heart failure caused by left ventricular systolic dysfunction [Witte 2005].
2006: Stranges does a secondary analysis of the effect of selenium on cardiovascular incidence and mortality
Stranges et al went back and re-examined the data from the entire blinded phase of the Nutritional Prevention of Cancer Trial (1983-1996), looking for data on the incidence and mortality of cardiovascular disease among the study participants who were free of heart disease at baseline. The existing NPC data did not show a significant association between selenium supplementation and the prevention of cardiovascular disease [Stranges 2006].
2008: Bekaert and Rayman investigate selenium’s effect on homocysteine
Homocysteine in high levels has been directly associated with increased risk of cardiovascular disease. Its status in plasma is known to be inversely associated with plasma selenium status. Consequently, in one of the sub-studies of the double-blind, placebo-controlled UK PRECISE pilot study, Bekaert and Rayman and their colleagues investigated the interaction between selenium, homocysteine, and B vitamins.
The researchers randomly assigned 501 healthy elderly British volunteers to one of four study groups for a six-month period:
- 100 micrograms of high selenium yeast SelenoPrecise® tablets per day
- 200 micrograms of high selenium yeast SelenoPrecise® tablets per day
- 300 micrograms of high selenium yeast SelenoPrecise® tablets per day
- Matching placebo tablets
The study data show that, at baseline, selenium status was, in fact, inversely correlated with total homocysteine status but only in male participants. At the end of the six-month study period, only selenium status had changed significantly. The supplementation with selenium did not affect total homocysteine levels in elderly UK elderly volunteers [Bekaert 2008].
2010: Leong and Rosenfeldt report on antioxidant therapy before and after heart surgery
Leong, Rosenfeldt, et al randomly assigned 117 patients scheduled for elective coronary artery bypass graft (CABG) and/or valve surgery to an adjuvant treatment group receiving antioxidant supplements or to a placebo control group. For approximately two months prior to surgery and one month following surgery, the patients in the adjuvant treatment group received the following supplements:
- Selenium
- Coenzyme Q10
- Magnesium orotate
- Lipoic Acid
- Omega-3 fatty acids
The patients had a mean age of 65 years. The ratio of male to female patients was 3:1.
The rationale for the study was the researchers’ observations that heart surgery increases measures of biomarkers of oxidative stress and reduces plasma antioxidant levels. In this study, the use of the antioxidant supplement therapy increased antioxidant levels and attenuated the adverse effects of surgery that are related to oxidative damage. The use of the antioxidant supplements also shortened the average length of postoperative hospital stays by 1.2 days.
Best of all, the antioxidant supplement therapy was inexpensive and had no clinically significant side effects. It reduced the extent of the damage caused by surgery to the heart muscle, and it shortened the length of the hospital stay following surgery [Leong 2010].

Illustration: Dr. Franklin L. Rosenfeldt, research professor at Monash University and cardiothoracic surgeon at Alfred Hospital, Melbourne, Australia. Dr. Rosenfeldt has long been interested in the use of antioxidant supplements such as selenium and Coenzyme Q10 to improve to improve the heart function of patients before and after surgery.
2011: Rayman tests the effect of supplementation with high-selenium yeast on plasma lipids
Rayman et al carried out an analysis of the data from the randomized, placebo-controlled, parallel-group UK PRECISE pilot study. She used data stratified by age and sex. All participants and researchers and evaluators were blinded to treatment or placebo assignment. For six months, 501 volunteer participants aged 60 to 74 years were assigned to one of four groups;
- Receiving 100 micrograms per day of SelenoPrecise® high-selenium yeast
- Receiving 200 micrograms per day of SelenoPrecise® high-selenium yeast
- Receiving 300 micrograms per day of SelenoPrecise® high-selenium yeast
- Receiving a yeast-based placebo for 6 months
At baseline and again at six months, the researchers measured total and high- density lipoprotein (HDL) cholesterol concentrations. They calculated the non-HDL cholesterol levels.
In the groups receiving the daily high-selenium yeast supplements, the mean plasma selenium concentrations increased significantly. Selenium supplementation was reported to have modestly beneficial effects on total cholesterol, HDL cholesterol, and non-HDL cholesterol levels. The ratio between total cholesterol and HDL cholesterol also improved with selenium supplementation.
The British researchers noted that the clinical significance of the effect of selenium supplementation on plasma lipids remains unclear. In particular, it is not clear whether the effect of selenium supplementation is beneficial only in individuals with low selenium status at baseline or is equally beneficial in individuals with relatively high selenium status at baseline.
The mean plasma selenium status at baseline in the UK PRECISE study was 91 micrograms per liter. A normal selenium status is thought to be 100 micrograms per liter. Accordingly, selenium status in the range of 50 – 80 micrograms per liter would be considered low; selenium status in the range of 100 – 120 micrograms per liter would be considered relatively high [Rayman 2011].
2013: Fedacko investigates the effect of combination selenium and Coenzyme Q10 on statin-associated myopathy
Fedacko et al carried out a 3-month randomized, double-blind, placebo-controlled trial in which 60 eligible patients reporting statin-associated myopathy (muscle pain, muscle weakness, tiredness, or muscle cramps) were assigned to one of four study groups:
- group getting 200 micrograms of SelenoPrecise® high selenium yeast tablets + 200 milligrams of Bio-Quinone Q10 capsules daily
- group getting a selenium placebo + 200 milligrams of Bio-Quinone Q10 capsules daily
- group getting 200 micrograms of SelenoPrecise® high selenium yeast tablets + Q10 placebo daily
- group getting a selenium placebo + a Q10 placebo daily
The study results showed that Q10 supplementation of statin-treated patients significantly diminished the symptoms of statin-associated myopathy. Selenium supplementation alone of statin-treated patients was not associated with clinically significant benefits in reducing the symptoms of statin-associated myopathy. However, the researchers noted that there is probably some association between selenium availability and Coenzyme Q10 deficiency. In this study, they observed a higher increase of Q10 levels in the group that was supplemented with Q10 together with selenium [Fedacko 2013].
2013: Bogsrud reports no significant effect of combination Coenzyme Q10 and selenium on statin-induced myopathy
The Norwegian researchers recruited 43 patients who had previous or ongoing statin-induced myopathy (muscle pain or weakness) while on atorvastatin therapy. After a 6-week washout period in which the patients received no statin medication, the patients were administered the corresponding placebos or a combination of 10 milligrams of atorvastatin, 400 milligrams of Coenzyme Q10, and 200 micrograms of selenium per day for 12 weeks.
Despite significant increases in the serum Q10 and selenium levels in the patients in the treatment, the researchers found no statistically significant improvements in the symptoms of statin-induced muscle pain and weakness relative to the patients in the placebo group.
However, four patients in the placebo group had to stop the atorvastatin treatment because of unbearable pain from the statin medication. No patients in the treatment group had to stop the statin treatment. This difference was not statistically significant, but it might be clinically significant. Furthermore, the researchers speculated that Coenzyme Q10 and selenium supplementation might be more important at higher dosages of atorvastatin [Bogsrud 2013].
2013: Rees does systematic review for the Cochrane Database
Rees et al did a systematic review of randomized controlled trials that tested the efficacy of selenium-only supplementation on cardiovascular risk. The researchers included 12 trials enrolling 19,715 participants [Rees 2013]. Selenium supplementation was seen to reduce
total cholesterol but not at a statistically significant level. In one study, selenium supplementation did significantly reduce the level of non-HDL cholesterol [Rees 2013]
Rees’ review included the selenium-only participants from the SELECT study who took a daily supplement of 200 micrograms of synthetic l-selenomethionine (not a high selenium yeast preparation).
Rees et al published their review before they got a chance to see the results from the KiSel-10 study carried out by Professor Alehagen and his colleagues in Sweden [Alehagen 2013, 2015]. Given the limitations of the clinical trial evidence available to them, the Cochrane review did not support the use of selenium supplements for the primary prevention of cardiovascular disease [Rees 2013]. Even though the KiSel-10 study was not a selenium-only study, the data from that study carried out by Alehagen et al would seem to force a re-consideration.
2013 – 2015: Alehagen reports beneficial effects of combination Coenzyme Q10 and selenium supplementation in healthy elderly subjects
Professor Urban Alehagen and a group of researchers at Linköping University in Sweden had seen that otherwise healthy elderly people with low selenium status (67 micrograms per liter on average) are at significantly higher risk of dying from cardiovascular disease. The Swedish researchers regarded such low selenium levels as below the physiological saturation level for several selenoprotein enzymes. [Alehagen 2016].
In the main report on the results of their KiSel-10 study, Alehagen et al reported that daily supplementation with 200 micrograms of SelenoPrecise® and 200 milligrams of the Coenzyme Q10 preparation Bio-Quinone Q10 Gold for a five-year period significantly lowered the risk of dying from cardiovascular disease by 54% as compared to participants in a placebo group [Alehagen 2013].
All of the participants in the KiSel-10 study were healthy elderly individuals who, nevertheless, did have more heart issues at the start of the study than would be seen in groups of younger individuals. The supplementation with a combination of selenium and Coenzyme Q10 slowed the normal decline in heart function that comes with increasing age.
The KiSel-10 study data showed an improvement in a bio-marker for the existence and severity of heart failure, the NT-proBNP bio-marker [Johansson 2013]. Furthermore, the study data showed decreased levels of bio-markers for inflammation, oxidative stress, and atherosclerosis [Alehagen 2015].
Further analysis of the data from the KiSel-10 study showed an increased quality of life and a reduced number and duration of stays in a hospital for those participants who received the active treatment with the combination of selenium and Coenzyme Q10 [Johansson 2015].
Interestingly, when the Swedish researchers did a 10-year follow-up of the participants using the Swedish National Register of Mortality based on death certificates and autopsy reports, they found a statistically significant lasting protection of SelenoPrecise® and Bio-Quinone Q10 Gold supplementation as compared with placebo. Even ten years after the participants’ baseline, there was a significant reduction in cardiovascular mortality and in all-cause mortality [Alehagen 2015]. The researchers suggested that the reduction in mortality in the elderly sample could be attributed to the reductions in inflammation associated with the use of the combination supplementation.

Illustration: Professor Urban Alehagen, senior physician and associate professor of cardiology at Linköping University in Sweden, was the lead researcher on the KiSel-10 randomized controlled trial.
Evaluating the KiSel-10 study results from the point of view of a clinician, Professor Alehagen made the following observations [Alehagen 2015]:
- There is an important interrelationship between Coenzyme Q10 and selenium. Selenium deficiencies can inhibit the cells from getting optimal concentrations of Coenzyme Q10, and adequate concentrations of Q10 must be available for the cells to benefit from optimal selenium function.
- Coenzyme Q10 (in its reduced form) is an important antioxidant, and selenium is an essential component of important antioxidant enzymes. Antioxidant protection is especially important in the prevention of heart disease because the mitochondrial DNA in the heart muscle cells are especially susceptible to damage caused by oxidative stress.
- There is an interrelationship between selenium and Coenzyme Q10 that can be exploited for therapeutic advantage if both substances are used together as a preventive measure in middle-aged and elderly persons at risk for developing heart disease and as an adjunctive treatment of patients diagnosed with heart failure.
It is interesting to see that the percentage of reduction in the risk of cardiovascular death with supplementation corresponds closely to percentage of increased risk of
cardiovascular death in individuals with low selenium status prior to supplementation. One interpretation is that the supplementation with selenium led to a normalization of bodily functions that are dependent upon the activity of various selenoproteins.
2015: Cold reports on the effect of selenium on cholesterol in Danish PRECISE pilot study
The Danish PRECISE (PREvention of Cancer by Intervention with SElenium) pilot study was a 5-year randomized, double-blind, placebo-controlled trial with men and women aged 60 – 74 years assigned to four groups:
- 100 micrograms of SelenoPrecise® per day
- 200 micrograms of SelenoPrecise® per day
- 300 micrograms of SelenoPrecise® per day
- Matching placebo each day
The researchers measured plasma samples for selenium status and total cholesterol and HDL-cholesterol at baseline, six months, and five years. Supplementation increased selenium status significantly and in dose-dependent fashion. However, the increases in plasma selenium levels were not associated with changes in cholesterol levels that were significantly different from the changes in cholesterol levels in the placebo group.
2016: Zhang does a meta-analysis of the selenium and cardiovascular disease research literature
Zhang et al included 16 observational studies and 16 randomized controlled trials in their review. The analysis of the observations showed a significant benefit for cardiovascular health in the selenium status range of 55-145 micrograms per liter, that is, there were clear beneficial health effects in individuals with selenium status ranging from quite low levels to levels clearly above normal status.
The analysis of the randomized controlled trials showed no significant effect on cardiovascular health. However, the researchers did not include the results of the KiSel-10 study enrolling 443 participants conducted by Alehagen and colleagues. The researchers concluded that their findings indicate the need for more study of the appropriate selenium status, selenium supplement dose, and selenium safety.
What have we learned from the selenium and cardiovascular disease clinical trials?
The best summary of what we know is the summary published by Alehagen and Aaseth [2015]:
- Selenium is a known essential component of several important antioxidant enzymes. Coenzyme Q10 in its reduced form is a known important fat-soluble antioxidant. Antioxidant protection is an important aspect of the prevention of cardiovascular disease.
- There exists an interrelationship between selenium and Coenzyme Q10 and their biological functions that needs additional research. Funding for this research is difficult to obtain because the use of the two substances for medicinal purposes cannot be patented and exploited.
- The combined use of selenium supplements at the level of 200 micrograms per day and Coenzyme Q10 at the level of 200 milligrams per day can have preventive benefits for healthy individuals.
Selenium and Thyroid Hormone Regulation
Selenium is known to be more concentrated in the thyroid gland than in any other organ in the human body. It is known to be a necessary component for the conversion of the inactive T4 thyroid hormone into the active T3 thyroid hormone. The beneficial effects to the thyroid gland of supplementation with selenium are thought to be improved conversion from T4 to T3 thyroid hormone and protection of the thyroid gland cells against oxidative damage, in particular from the harmful effects of the hydrogen peroxide radical [Rayman 2000].
2011: Marcocci reports on selenium supplementation and well-being of Graves’ disease patients
In a randomized controlled trial lasting 6 months, Marcocci et al reported that Graves’ disease patients given selenium (100 micrograms twice daily) achieved a significantly improved quality of life, significantly reduced less eye movement, and significant slowing of the progression of Graves' orbitopathy, as compared with the placebo group. There were no adverse events associated with the selenium supplementation.
2013: Bülow Pedersen reports low levels of serum selenium in Graves’ disease
The researchers measured serum selenium status in the following four study groups:
- Patients with newly diagnosed Graves' disease (n = 97)
- Patients with autoimmune overt hypothyroidism (n = 96)
- Subjects with normal thyroid function but high thyroid peroxidase antibody levels (n = 92)
- Random controls (n = 830)
The serum selenium levels were significantly lower in patients with Graves’ disease than in the controls, also when the results were adjusted for age, sex, mineral supplements, smoking, geographical region, and time of sampling. There was no similar difference between the serum selenium levels of subjects with normal thyroid function and the random controls.
The researchers concluded from the results of the study that serum selenium status is lower in patients with newly diagnosed Graves’ disease and autoimmune overt hypothyroidism compared with random controls and compared with subjects with normal thyroid function. Their data suggest a link between inadequate selenium status and Graves’ disease and overt autoimmune thyroid disease [Bülow Pedersen 2013].
2013: Van Zuuren does Cochrane review of efficacy of selenium supplementation in Hashimoto thyroiditis
The Cochrane researchers reviewed the results of four randomized controlled trials enrolling a total of 463 patients. The results from the four trials did not provide conclusive evidence that selenium supplementation can raise the levels of thyroid hormones sufficiently that hormone replacement therapy with levothyroxine (LT4) could be decreased or discontinued [van Zuuren 2013]. Three of the four studies reviewed indicated that selenium supplement resulted in a reduction in thyroid peroxidase antibodies [Krysiak 2011, Negro 2007, Turker 2006].
Clearly there is a need for the funding of additional trials of the efficacy of selenium supplementation in Hashimoto thyroiditis, which causes inflammation and tissue damage
and hypothyroidism. In particular, studies that address the effect of selenium supplementation on mood and quality of life are needed.
2013: Drutel reviews the state of selenium and thyroid hormone research
Drutel et al reviewed the research literature on the effects of selenium supplementation for thyroid patients. They found that the effect of selenium supplementation on the immune system is most often attributed to the neutralization of free radicals and their metabolites.
The main points of the literature review included the following results:
- Selenium supplementation decreased anti-thyroid antibody levels and improved the ultrasound structure of the thyroid gland in patients with Hashimoto's disease and in pregnant women with anti-TPO antibodies.
- Selenium supplementation resulted in a return to normal thyroid function more quickly in patients with Graves’ disease.
- Selenium supplementation had a beneficial effect on mild inflammatory orbitopathy.
2013: Watt initiates the GRASS trial of selenium supplementation in patients with Graves’ disease
In 2013, Watt et al began recruiting Graves’ disease patients, patients with an autoimmune disorder that results in the overproduction of thyroid hormones (hyperthyroidism). The researchers intend to recruit 492 participants who are 18 years of age or older and who have been diagnosed with active Graves’ hyperthyroidism within the previous two months.
The participants will be randomized to a selenium supplementation group (two tablets of 100 micrograms of selenium daily) or to a placebo group for a trial period of 24-30 months. The primary outcome of the GRASS trial will be the extent of success or failure of selenium supplementation in improving the probability of the patients’ continued remission following cessation of anti-thyroid drug treatment. Secondary outcomes will be assessments of thyroid-related quality of life, measurements of the levels of thyroid stimulating hormone- receptor antibodies, hyperthyroid symptoms, and adverse events during the supplementation period [Watt 2013]
2014: Winther initiates the CATALYST study of the effect of selenium on chronic autoimmune thyroiditis
CATALYST stands for Chronic Autoimmune Thyroiditis Quality of Life Selenium Trial. Winther et al intend to recruit 2 x 236 patients for a randomized, double- blind placebo-controlled, multi-center study lasting 12 months. The recruited patients will have to meet the following inclusion criteria:
- 18 years of age or older
- Serum thyroid peroxidase antibody levels at or above 100 IU/milliliter
- Treatment with levothyroxine
The patients in the CATALYST trial will follow the conventional treatment at their usual hospitals. Patients in the selenium intervention group will take 200 micrograms of SelenoPrecise® high selenium yeast tablets daily. Patients in the control groups will take matching placebo tablets.
The primary endpoint of the CATALYST trial will be thyroid-related quality of life assessed by the Thyroid Patient-Reported Outcome (ThyPRO) questionnaire. Secondary endpoints will be serum thyroid peroxidase antibody concentrations, serum triiodothyronine/thyroxine ratios, levothyroxine dosages, and adverse reactions and events [Winther 2014].
2015: Winther reports on an investigation of the effect of selenium on thyroid function
The thyroid gland continues its selenium and selenoprotein activity even in individuals with severe selenium status deficiencies [Kohrle 2005]. Because selenium is important for thyroid hormone synthesis and metabolism, Winther et al investigated the effect of different doses of selenium on thyroid function in study participants with low selenium status and no known thyroid disorder. The researchers randomly assigned 491 males and females aged 60-74 years to one of four groups:
- 100 micrograms per day
- 200 micrograms per day
- 300 micrograms per day
- matching yeast-based placebo tablets
The supplement used in all three intervention groups is the high selenium yeast
SelenoPrecise® provided by Pharma Nord. Altogether, 361 participants, equally distributed in the four study groups, completed the five-year randomized, double- blind, placebo-controlled trial.
The researchers analyzed the participants’ plasma samples for selenium levels and analyzed serum samples for thyroid-stimulating hormone (TSH), free triiodothyronine (FT3 hormone), and free thyroxine (FT4 hormone) at baseline, after 6 months, and after 5 years of supplementation.
The study data showed that plasma selenium concentrations increased significantly and in a dose-dependent manner in all three intervention groups receiving selenium. At the same time, the serum TSH and FT4 concentrations decreased significantly and in a dose-dependent way. Moreover, there were no significant differences between the serum concentrations at 6 months and at 5 years.
The study data did not show any significant effects on FT3 concentrations or on the ratio of FT3 hormone to FT4 hormone. The researchers concluded that thyroid function in individuals with normally functioning thyroid glands and adequate selenium status is probably affected to some small extent through the reduction serum TSH and FT4 concentrations. Still undecided is the role of selenium supplementation in individuals with a severe selenium deficiency and, more importantly, in individuals being treated for autoimmune thyroid diseases [Winther 2015].
Danish PRECISE study results different from UK PRECISE study results vis-à-vis thyroid function
The results of Winther’s Danish PRECISE study showed that selenium supplementation did affect thyroid function in a dose-dependent manner; selenium supplementation decreased serum TSH and FT4 concentrations in elderly Danes with no known thyroid disorders. The Danish PRECISE data on the effect of selenium supplementation on thyroid function differ from the data of Rayman’s earlier analysis of the UK PRECISE study [Rayman 2008]. Rayman’s data did not establish any significant effect on thyroid function or thyroid hormone conversion. One possible explanation is that the Danish PRECISE study data were drawn from a clinical trial that had a larger sample size and that lasted longer (five years). Furthermore, the results may have been affected by the mandatory iodization of table salt in Denmark, which could have affected selenium-iodine interactions in the Danish study participants [Winther 2015].
What have we learned from the selenium and thyroid function clinical trials?
The role of selenium supplementation in the treatment of autoimmune thyroid diseases is still unresolved. The forthcoming results of the GRASS trial and the CATALYST trial may provide significant evidence for an effect of selenium supplementation on thyroid function.
Selenium and Defense against Heavy Metals
Selenium and selenoproteins bind with heavy metals such as cadmium, lead, and mercury and help to remove these neurotoxic substances from the body. There is, however, a cost involved. One the one hand, the removal of heavy metals by binding to selenium is an efficient detoxification mechanism; on the other hand, the process of removing heavy metals from the body results in the physiological inactivation of the selenium involved in the detoxification. Consequently, there is less selenium (and fewer selenoproteins) available to provide antioxidant, anti- mutagenic, anti-viral and anti-carcinogenic defenses in the body [Schrauzer 2009].
Mercury
Methylmercury is a highly toxic form of mercury; it is the most common form of mercury encountered by humans. Fish are the major source of methylmercury in the diet.
Primarily from animal studies, we know that selenium status is inversely associated with methylmercury toxicity. Moreover, we know that exposure to methylmercury is less serious in animals that have been fed a selenium-enriched diet. Animal studies have shown that selenium can not only prevent methylmercury toxicity but can also reverse some of its symptoms but only at the cost of reduced bioavailability of selenium [Ralston & Raymond 2010].
It has been estimated that mercury’s affinity for binding with selenium is approximately a million times higher than mercury’s affinity for binding with sulfur, which is its second-best binding partner. Methylmercury naturally sequesters selenium, which directly impairs beneficial selenoenzyme synthesis and beneficial selenoenzyme activities [Ralston & Raymond 2010].
Among other things, the sequestering of selenium by methylmercury impairs the antioxidant activities of the selenoenzymes. Moreover, mercury crosses the blood-brain barrier with relative ease, and, given its high affinity for selenium, mercury sequesters the selenium in the brain and reduces the extent of selenoprotein synthesis in the brain.
So, selenium definitely has a protective effect against mercury in that it forms molecular complexes with the mercury that keep the mercury from having toxic effects on brain tissue. But, the bioavailability of selenium for other functions is reduced accordingly [Ralston & Raymond 2010]. Consequently, in cases of mercury exposure, a steady supply of dietary and supplemental selenium is needed.
High maternal exposures to methylmercury can inhibit selenium-dependent enzyme activities in fetal brains. Augmented maternal dietary and supplemental selenium intakes are needed to preserve the selenium-dependent enzyme activities in the mother and in the fetus to prevent pathological outcomes [Berry & Ralston 2008].
Fish as a source of mercury
Fish are generally recognized as the major source of mercury in the diet. Fish is a good source of protein, is low in saturated fat, and is a primary source of omega-3 fatty acids; fish should be part of a balanced diet. However, almost every species of edible fish contains at least traces of methylmercury.
Some research suggests that most fish species provide humans with as much selenium as mercury, measured in molar concentrations of the selenium and the mercury [Kaneko & Ralston 2007]. The thinking is that eating fish with a more or less equal ratio of selenium to mercury affords protection against the harmful effects of the ingested mercury. However, it can be seen that the selenium gained in the diet from fish does not provide extra selenium for selenoprotein synthesis and activity.
Eating lots of fish does not seem to be a substitute for taking a selenium supplement.
The US Food and Drug Administration (FDA) specifically warns against eating four species of fish that are known to contain high levels of mercury in excess of selenium:
- Shark
- Swordfish
- King Mackerel
- Tilefish
The FDA has also cautioned against eating more than one serving (6 ounces) of canned tuna per week [What You Should Know 2014].
Amalgam fillings as a source of mercury
The question of the extent to which mercury vapor is released from amalgam fillings during normal activities such as chewing and brushing is perhaps the second most controversial issue in modern dentistry, following only the issue of fluoride. Some studies have shown that the level of mercury vapor that is released from amalgam fillings is below the level that causes harm to tissue in the body and the brain; however, there are still concerns about the accumulation of mercury from dental amalgam over a number of years and about an apparent decreasing ability to eliminate mercury with increasing age [Weiner 1990].
Fortunately, the use of dental amalgam is declining, but many people have amalgam fillings, and, there now seems to be reason to believe that individuals respond differently to mercury exposure [Parajuli 2016]. Similarly, it seems plausible that individuals with health issues may have a different selenium metabolism from healthy individuals.
There still exists a need to collect and analyze more data on the health risk of mercury exposure from dental amalgam, in particular data from longitudinal studies. There seems to be some evidence that amalgam fillings continuously release small amounts of mercury in the form of mercury vapor, ions, and fine particles [Bates 2006, Weiner 1995]. The released mercury vapor is absorbed to a considerable degree by the lung tissue [Weiner 1990].
To get an estimate of the extent of the problem, Nylander et al analyzed the mercury concentrations in the human brain and kidneys in autopsy samples. They found a statistically significant positive correlation between the number of amalgam fillings and the concentration levels of mercury in the occipital lobe cortex. They also found a significantly higher mercury level in the kidney cortex of individuals with amalgam fillings than in individuals with no fillings. The researchers suggested that one explanation would be the release and accumulation of mercury vapor from amalgam fillings [Nylander 1987].
Akesson et al reported that urinary mercury levels and plasma mercury levels were significantly higher in 244 dental personnel (who handled amalgam regularly) than in 81 matched controls. They also found that the amount of amalgam in the mouths increased the levels of mercury in whole blood, plasma, and urine. The greater the amount of amalgam in the mouth, the greater the increase in mercury levels [Akesson 1991].
2000: Seppänen investigates the effect of selenium supplementation on the accumulation of mercury in the body
Seppänen et al investigated the effect of a yeast-based selenium supplement on selenium status and mercury status in subjects with low serum selenium. The study enrolled 23 participants with a below average serum selenium status of less than 90 micrograms per liter; the study lasted four months. 13 of the participants were randomly assigned to the selenium supplement group; 10 were randomly assigned to the placebo group.
The participants in the selenium supplementation group received 100 micrograms of selenium daily. The results of the study showed that selenium supplementation raised the serum selenium levels by 73% and the blood selenium levels by 59% and reduced the mercury levels in the pubic hair of the participants by 34%. The researchers concluded that relatively small doses of supplemental selenium reduce the accumulation of mercury in pubic hair in a relatively short time [Seppänen 2000].
2012: Li reports on the effects of selenium supplementation in long-term mercury-exposed populations
Residents of Wanshan, China, were known to suffer from elevated exposure to environmental mercury. Li et al recruited 103 local residents and randomly assigned 53 to supplementation with 100 micrograms daily of a SelenoPrecise® selenium- enriched yeast preparation; they assigned 50 local residents to supplementation with a non-selenium-enriched yeast placebo. The trial lasted for 3 months.
The researchers compared the effects of the selenium supplementation with the effects of the placebo supplementation on urine mercury levels, selenium status, and levels of biomarkers for oxidative stress. This 3-month supplementation with a high selenium yeast preparation showed that the organic selenium supplementation with SelenoPrecise® increased the elimination of mercury and decreased the levels of malondialdehyde and 8hydroxy-2-deoxyguanosine levels – bio-markers of oxidative stress – in the urine [Li 2012].
Cadmium
Cadmium is a toxic heavy metal that accumulates in the body from various sources, both environmental and nutritional. Inhaled cigarette smoke is a known source of cadmium. Cadmium causes damage to the blood, bones, kidney, liver, and lungs.
According to Schrauzer [2009], cadmium has several other toxic effects in the body:
- Increases the risk of breast cancer
- Increases the risk of prostate cancer
- Alters the metabolism of steroid hormones
- Disrupts the antioxidant enzyme system
- Impairs the function of the p53 protein (the protein that regulates the cell cycle and helps to prevent genome mutation)
Selenium has a high affinity for cadmium and binds with it to form a selenium-cadmium-protein complex. However, selenium can only protect against the harmful effects of cadmium to the extent that there is more selenium than cadmium available.
Furthermore, the physiological inactivation of selenium in the process of sequestration of cadmium limits the ability of selenium to provide chemo-protection against cancer [Schrauzer 2009].
1989: Wei shows the influence of selenium on cadmium concentration and excretion
Wei et al randomly assigned 38 trial participants to a group that took a selenium supplement (150 micrograms daily for 21 days) or to a placebo group. The supplementation with selenium was associated with a marked decrease in red blood cell cadmium levels. Cadmium was mainly excreted in the feces following selenium supplementation.
One week after the discontinuation of the selenium supplementation, the elevated cadmium content in urine and feces decreased to control levels. Most of the studies of the protective effects of selenium against the harmful effects of cadmium have been observational studies of selenium status and cadmium toxicity and animal studies of the effects of supplementation of cadmium exposed rats with selenium.
What have we learned from the selenium and heavy metals clinical trials?
Selenium supplementation is associated with the increased elimination of heavy metals from the body. However, the binding of selenium to heavy metals necessarily results in a decrease in the amount of selenium available in the body for antioxidant and anticarcinogenic purposes.
Selenium and Neurodegenerative Diseases
The role of selenium supplementation in the prevention and treatment of neurodegenerative disorders has been only incompletely researched in clinical trials.
In terms of priority for the uptake and retention of selenium, the brain selenium levels remain comparatively stable even during times of selenium deficiency. Furthermore, the central nervous system as a whole seems to have the highest priority for the uptake and retention of selenium, ranking as high as the reproductive system and the endocrine system. Current research results seem to indicate that selenoprotein P plays a major role in the maintenance of selenium levels in the brain [Cardoso 2015].
Because the brain consumes oxygen and produces harmful free radicals (known as reactive oxygen species) as by-products, the brain needs antioxidant selenoenzymes to prevent and lessen the impact of oxidative damage. Cardoso et al regard the role of the antioxidant selenoproteins in the central nervous system as well-established [Cardoso 2015].
Alzheimer’s disease
Oxidative stress is thought to be central to the development of cognitive impairment in Alzheimer’s disease. In fact, oxidative stress – defined as an imbalance between harmful activity of free radicals and the body’s antioxidant defense mechanisms – is regarded as a major harmful factor in the development of many neurodegenerative disorders in addition to Alzheimer’s disease:
- amyotrophic lateral sclerosis
- Batten's disease
- multiple sclerosis
- Parkinson's disease
Oxidative stress degrades lipids, proteins, and nucleic acids. Oxidative stress results in structural and functional impairment of the cells and, ultimately, in the death of cells [Santos 2014].
Supplementation with vitamin and mineral antioxidant cocktails that include selenium have been shown to improve memory and cognitive function as shown in the following studies.
2011: Loef and Schrauzer report the results of a systematic review of the selenium and Alzheimer’s disease literature
Loef and Schrauzer evaluated 9 placebo-controlled studies, 4 prospective studies, 4 cross-sectional studies, 15 case control studies, 24 autopsy studies, and animal studies and cell culture studies. They did not find consistent clinical evidence that supplementation with selenium would be beneficial in the treatment of Alzheimer’s disease, but they did find a positive association of selenium status and cognitive function.
Furthermore, they uncovered findings from molecular biology that show an important role for selenium in the development of Alzheimer’s disease. They concluded that there is a need for large, long-duration clinical trials that could show the potential preventive effects of selenium supplementation [Loef 2011].
2011: Kesse-Guyot reports on the effect of a daily antioxidant combination on memory
Kesse-Guyot et al assessed the effects of a daily antioxidant nutrient supplement including 100 micrograms of high selenium yeast on the cognitive performance of the participants in the Supplementation in Vitamins and Mineral Antioxidants (SU.VI.MAX) study.
From 1994 to 2002, French researchers enrolled 4447 French participants aged 45-60 years in a randomized, double-blind, placebo-controlled study. The participants received the antioxidant nutrient combination including selenium or matching placebo. The median follow-up period in the Su.Vi.Max trial was 7.5 years.
The research data showed that the participants who received the active antioxidant supplement had significantly better episodic memory scores. However, the active antioxidant treatment improved verbal memory only in the participants who were nonsmokers and/or who had low serum vitamin C concentrations at baseline [Kesse-Guyot 2011].
2012: Scheltens shows improved memory function with an antioxidant combination including selenium
In the Souvenir II study, a 24-week, randomized, double-blind, controlled, parallel-group, multi-country clinical trial enrolling patients with mild Alzheimer’s disease, the patients in the active treatment took an antioxidant combination consisting of choline, fish oil, folate, vitamins B6, B12, C, and E, and 60 micrograms of selenium. The patients in the active treatment group increased significantly their memory function scores on the Neuropsychological Test Battery as compared with patients in the placebo group.
Moreover, the patients in the active treatment group achieved electroencephalogram (EEG) measures of functional connectivity that were significantly more favorable than the EEG measures in the placebo group [Scheltens 2012].
2014: De Waal reports the preservation of brain networks in patients with mild Alzheimer’s disease
De Waal et al followed 179 patients diagnosed with mild Alzheimer’s disease for 24 weeks in a randomized, double-blind, controlled, parallel-group, multi-country study. The active treatment consisted of daily supplementation with an antioxidant combination that included choline, fish oil, folate, vitamins B6, B12, C, and E, and 60 micrograms of selenium.
The study results suggested that the antioxidant combination preserved the organization of brain networks in the patients, inhibiting the progress over time of network disruption in Alzheimer’s patients [De Waal 2014].
2015: Cardoso shows that daily dietary supplementation with a Brazil nut improves cognitive performance
Supplementation with one Brazil nut daily for six months, providing a daily dose of approximately 288 micrograms of selenium, has also been associated with improved cognitive performance [Cardoso 2015]. However, many people do not have access to Brazil nuts on a daily basis.
Amyotrophic lateral sclerosis
Accumulation of free radicals and the resultant oxidative damage have been suggested as contributing factors in the development of amyotrophic lateral sclerosis (ALS). A recent study of blood trace element status in amyotrophic lateral sclerosis patients has shown that selenium status and ALS severity are inversely associated, suggesting that selenium supplementation might be beneficial [Peters 2016].
A systematic review of studies involving antioxidant supplementation of ALS patients found that there were only four possibly relevant studies to review, that all four studies were poorly designed and underpowered, and that the four studies failed to show a significant effect of antioxidant supplementation with l-methionine, Vitamin E, and selenium alone or in combination. Although there is no substantial evidence from clinical studies to support the use of antioxidants in ALS, there is also no clear contraindication, and selenium is an affordable supplement to conventional medication [Orrell 2008].
Batten’s disease
Jensen and Clausen found that the glutathione peroxidase activity was significantly reduced in whole lymphocytes from patients suffering from Batten's disease. They theorized that an increased peroxidation that damages cellular membranes contributes to the further development of Batten’s disease [Jensen & Clausen 1983]. Clausen and Jensen suggested that abnormal biochemical status in Batten’s disease patients could be normalized by an antioxidant supplement combination containing selenium [Clausen & Jensen 1988].
Huntington’s disease
At least one study has suggested that selenium levels in the plasma of Huntington’s disease patients do not differ from the selenium levels in the plasma of normal controls [Cardoso]. However, Pillai [2014] suggests that adequate levels of selenium can deter lipid peroxidation damage in Huntington’s disease by increasing specific glutathione peroxidases.
Multiple sclerosis
As in the case of Alzheimer’s disease, oxidative stress is thought to be a crucial factor in the development of multiple sclerosis (MS). Studies have shown that selenium status tends to decrease in patients with multiple sclerosis and the levels of glutathione peroxidase activity are decreased [Cardoso 2015]. There are no reports of results from clinical trials at the time of this writing (winter 2016). The focus in complementary and alternative medical therapies for multiple sclerosis seems to be on lipoic acid, omega-3 fatty acids, and vitamin D rather than on selenium [Yadav 2010].
Clausen, in an early open-label study, showed that supplementation with selenium and vitamin C and vitamin E could restore the lower blood selenium levels and lower glutathione peroxidase activity levels in MS patients to normal levels [Clausen 1988].
Parkinson’s disease
No clinical trials have established an association between selenium status and Parkinson’s disease. Shahar et al have been able to associate plasma selenium levels and performance-based assessments of coordination but have been unable to find any measurable association between plasma selenium levels and Parkinson’s disease [Shahar 2010].
What have we learned from the selenium and neurodegenerative diseases clinical trials?
Selenium metabolism undoubtedly plays a role in the central nervous system, in neurodegenerative diseases such as Alzheimer’s disease, and in cognitive function; however, the mechanisms are not well understood at this time, and not many clinical trials have been carried out.
Selenium and HIV/AIDS
HIV/AIDS
Selenium deficiency and low selenium status have been associated with HIV and AIDS in observational studies. Clinical trials have shown improvement of symptoms and survival in individuals infected with HIV. The exact mechanisms for the positive health effects of selenium supplementation as an adjunct to anti-retrovirus therapy are not known. One conjectured mechanism is the antioxidant protection of the action of the selenoproteins, in particular the glutathione peroxidases and the thioredoxin reductases.
The following randomized, double-blind, placebo-controlled studies have shown various significantly positive health benefits in HIV-infected samples.
2002: Burbano shows decreased numbers of hospital admissions with selenium supplementation
Daily supplementation for 2 years with 200 micrograms of selenium in addition to antiretroviral treatment in a study enrolling 186 HIV-positive patients reduced the rate of hospital admissions significantly as compared with placebo. As a consequence, hospitalization costs were also reduced significantly in the selenium supplementation group [Burbano 2002].
2007: Hurwitz reports reduced HIV virus progression and increased CD4 counts
Daily supplementation for nine months with 200 micrograms of a high selenium yeast supplement in addition to anti-retroviral treatment in a study completed by 174 HIV-infected patients showed significantly reduced HIV virus progression and increased CD4 counts. (CD4 cells are T-cells and T-helper cells that are the white blood cells protecting the body from infections.)
There were no recorded adverse effects associated with the selenium supplementation. The positive health effects of the selenium supplementation remained significant after the researchers had controlled for such confounding variables as age, current or past drug abuse, education level, ethnic background, gender, hepatitis C infection, HIV symptoms, income level, and time since HIV diagnosis [Hurwitz 2007].
2008-2009: Kupka reports selenium supplementation reduces risk of infant mortality in Tanzania
Daily supplementation of 913 pregnant Tanzanian women with 200 micrograms of selenium in the form of l-selenomethionine during the period from 12 – 27 weeks of gestation until 6 months following childbirth did not have an effect on T-cell counts or HIV viral progression but did significantly reduce the risk of acute and persistent diarrhea [Kupka 2009]. Moreover, selenium supplement significantly reduced the risk of infant mortality in the period between 6 weeks and 6 months following childbirth [Kupka 2008].
2013: Baum reports efficacy of micronutrient supplementation including selenium in HIV-positive adults in Botswana
Baum et al randomly assigned 878 HIV-positive adults in Botswana (who were not receiving anti-retroviral treatment) to one of four study groups for a 24-month trial period:
- B vitamins and vitamin C and vitamin E group
- Selenium group, 200 micrograms, in the form of selenomethionine
- Combined vitamins and selenium group
- Placebo group
The study data showed that supplementation with vitamins plus selenium slowed the decline of the patients’ immune system; supplementation with selenium alone or with vitamins alone did not produce results significantly different from the results in the placebo group [Baum 2013].
What have we learned from the selenium and HIV/AIDS studies?
Clinical trials of selenium supplementation in HIV/AIDS patients have shown such benefits as decreased hospitalization rates and costs, slowed progression of the virus, increased T-cell counts, decreased infant mortality for children of supplemented mothers, and decreased risk of diarrhea. Selenium supplementation continues to be a possible adjunct therapy in HIV-infected patients on anti-retroviral medications. More research into the mechanisms of selenium’s impact on HIV is needed [Stone 2010].
Stone et al [2010] in Table 3 of their review article about the role of selenium in HIV infection list the study sample size and characteristics, intervention type, and results for intervention studies with and without control groups.
Selenium and Oxidative Stress
Oxidative stress is a term used to describe the condition that arises whenever there is cell and tissue damage caused by a critical imbalance between the harmful free radicals and the neutralizing antioxidants in the body. Free radicals have an unpaired electron in their outer orbitals; consequently, they are independent and unstable and very reactive. Free radicals are generated by the body’s own metabolic processes that involve oxygen and by exposure to environmental factors such as air pollutants, cigarette smoking, industrial chemical, ozone, ultraviolet rays from sunlight, and X-rays. Oxidative damage is common in disease and injury.
In moderate numbers, free radicals perform useful functions in the body. However, if excessive numbers of free radicals are generated and are not neutralized by antioxidants, they can inflict widespread molecular damage on cells and tissues and cell DNA. Free radical damage has been associated with the development of many degenerative conditions including some cancers, heart disease, inflammatory conditions like arthritis, and ageing [Lobo 2010].
Some of the most common highly reactive oxygen free radicals are the hydrogen peroxide radical, the hydroxyl radical, the oxygen singlet radical, the peroxynitrite radical, and the superoxide anion radical. Selenium is a component of many important antioxidants in the body. Other important antioxidants are Coenzyme Q10, the vitamins C and E, and various carotenoids that are found in foods.
1992: Kuklinski tests the effects of selenium on UV light-induced oxidative stress
Kuklinski et al did a 14-day substance-comparison intervention study in 24 healthy volunteers. The researchers induced oxidative stress by exposing the volunteers to 15 minutes of ultraviolet light. The UV light exposure induced increases in the concentrations of the participants’ thiobarbituric acid reactive substances ((tbars = byproducts of lipid peroxidation). The increases lasted for one to two days. The researchers tested the effects of the following nutritional supplements, taken daily, on the oxidative stress:
- 200 micrograms of selenium in the selenomethionine form (Bio-Selenium)
- 600 milligrams of Vitamin E
- 45 milligrams of beta-Carotene
- 1 milliliter of Ginkgo biloba extract 3 times per day
The 14-day period of selenium supplementation was most effective at inhibiting the effect of exposure to ultraviolent light, followed in order by the Ginkgo biloba extract, the beta-carotene supplement, and the vitamin E supplement.
1997: Hussein evaluates the effect of selenium on oxidative damage in kidney transplant patients
Hussein et al undertook a 6-month intervention study with 6 kidney transplant recipients who were subject to elevated oxidative stress and at high risk of developing atherosclerosis. The patients took 200 micrograms of selenium in the form of selenomethionine (Bio-Selenium) daily for three months and then took a placebo tablet for comparison purposes for three months.
The add-on treatment with selenium reduced the patients’ plasma lipid peroxidation levels by 50%. Then, during the placebo phase of the study, the patients’ plasma lipid peroxidation levels returned to baseline.
In the same way, the selenium supplementation reduced copper sulfate-induced LDL oxidation by 15%. Selenium treatment significantly raised the levels of the antioxidant enzyme glutathione peroxidase in the patients’ red blood cells and significantly reduced the levels of oxidized glutathione content in the patients’ red blood cells.
When, after three months, the selenium treatment was terminated, and the patients ingested placebo tablets for 3 months, all of the values of the glutathione system returned to baseline levels.
Selenium supplementation activates the glutathione system and acts as a potent antioxidant against plasma and LDL lipid peroxidation in kidney transplant recipients. In providing protection against LDL lipid peroxidation, selenium protects against the development of atherosclerosis.
2002: El-Bayoumy tests whether selenium supplementation reduces oxidative damage
El-Bayoumy et al conducted a randomized, double-blind, placebo-controlled trial enrolling 36 healthy adult males aged 19 – 43 (11 African Americans and 25 white Americans). Participants in the active treatment group took 247 micrograms of a high selenium yeast preparation daily for nine months. After nine months, all participants took a placebo preparation for three months.
The researchers took blood and urine samples at 3, 9, and 12 months. Plasma selenium levels in the participants taking the active preparation were double the baseline level at both 3 and 9 months. At the 12-month point, these plasma selenium levels were 136% of baseline. Plasma selenium levels remained the same in the placebo group all the way through from baseline to 12 months.
In the selenium group participants, the researchers found a statistically significant 32% increase in blood glutathione levels at 9 months. There were also significant a 26% decreases in protein-bound glutathione and 44% decreases in the ratio of protein-bound glutathione to glutathione. There was no glutathione change in the placebo group participants.
The changes in glutathione and protein-bound glutathione correlated highly with the participants’ changes in plasma selenium concentrations. The researchers thought that the glutathione changes reflected a decrease in oxidative stress.
Additionally, in the selenium treatment group, the researchers noted a small but significant decrease in prostate-specific antigen levels after 3 and 9 months. This difference disappeared after the transition to three months on placebo [El- Bayoumy 2002].
2008: Skesters evaluates the effects of selenium in Chernobyl catastrophe clean-up workers
Skesters et al did a year-long randomized, double-blind, placebo-controlled study of the effects of selenium supplementation in 134 Chernobyl catastrophe clean-up workers, aged 43–55 years, who were at increased risk of developing cancer. The workers in the active treatment groups took 200 micrograms of SelenoPrecise® high selenium yeast alone or in combination with 100 milligrams of Bio-Quinone Q10, additional antioxidants, and ibuprofen per day. The workers in the control group took matching placebos.
The study data showed that the increased selenium status, vitamin E status, and total antioxidant status in the workers in the active treatment groups were associated positively with the following outcomes:
- improved anti-oxidative defense and improved quality of life
- decreased levels of lipid peroxides and oxidative stress
- decreased needs for prescribed medicines for joint pain, chronic bronchitis,
- emphysema, stomach troubles, and depression
- no incidence of new or abnormal growths compared to seven new cases of neoplasia in the control group
2008: Westermarck investigates the effect of selenium supplementation of HIV-infected outpatients on antioxidant status
Westermarck et al carried out a 6-week double-blind, placebo-controlled clinical trial with
24 male HIV-infected outpatients and 10 uninfected male controls of a similar age to the HIV-infected volunteers. The patients receiving the active treatment took 100 micrograms of SelenoPrecise® high selenium yeast and 100 milligrams of Bio-Quinone Q10 per day.
In this study, the HIV-infected patients had low baseline serum selenium (defined as a serum level ≤ 85 micrograms per liter). In epidemiological studies, selenium deficiency correlates positively with the progression and mortality of HIV infections. Selenium is necessary for the proper functioning of the immune system. It seems to be a key nutrient in the inhibition of HIV infection to the development of AIDS.
Antioxidant status improved in the active treatment group. Moreover, serum concentrations of selenium increased in conjunction with Coenzyme Q10 supplementation.
2008: Larmane reports on the effects of antioxidant supplementation on oxidative stress
Larmane et al conducted a 12-month placebo-controlled intervention study in 80 patients with reduced antioxidative defense system activity. The patients in the active treatment group received 100 micrograms of SelenoPrecise® high selenium yeast and 350 milligrams of Vitamin E daily.
The long-term supplementation significantly increased the patients’ plasma selenium and vitamin E levels. The selenium supplementation improved various lipid peroxidation parameters and significantly reduced lipid peroxidation activities.
2008: Voicehovska assesses oxidative stress parameters in bronchial asthma patients after selenium supplementation
Voicehovska et al did a 16-week intervention study with 20 patients with selenium deficit and a diagnosis of bronchial asthma (7 men and 13 women). The patients received a daily dose of 200 micrograms of SelenoPrecise® high selenium yeast. The supplementation of bronchial asthma patients with the high-selenium yeast resulted in a statistically significant increase of plasma selenium and resulted in a significant increase in selenium-dependent glutathione peroxidase activity. Using parameters of chemiluminescence, the researchers showed that the occurrence of oxidative stress in the blood of bronchial asthma patients is unusually high and that the treatment with selenium reduced the oxidative stress to normal values. The replenishment of the selenium deficiency in the bronchial asthma patients reduced oxidative stress and reduced the chronic inflammation process responsible for the development of bronchial asthma.
2012: Li assesses the effect pf selenium on bio-markers of oxidative stress
Li et al conducted a 3-month randomized, double-blind, placebo-controlled trial with 103 volunteers with long-term mercury exposure. The patients were given 100 micrograms of SelenoPrecise® high selenium yeast tablets daily. The researchers assessed the effects of selenium supplementation on the levels of the oxidative stress-related biomarkers malondialdehyde and 8-hydroxy-2-deoxyguanosine. The study results showed that SelenoPrecise® supplementation significantly decreased urinary malondialdehyde and 8hydroxy-2-deoxyguanosine levels in people with long-term mercury exposure.
2014: Richie tests the effect of two different forms of selenium on bio-markers of oxidative stress
Richie et al did a randomized, double-blind, placebo-controlled study of the effects of l-selenomethionine and high selenium yeast on cancer-relevant bio-markers of oxidative stress.
The researchers administered 200 micrograms of high selenium yeast, 285 micrograms of high selenium yeast, 200 micrograms of l-selenomethionine, or placebo to 69 health men. The study lasted for nine months followed by a three-month wash-out.
The dosage and form of the selenium in the 200-microgram high selenium group and the 200-microgram l-selenomethionine group were chosen to match the dosage and the form of selenium used in the Nutritional Prevention of Cancer trial [Clark 1996] and in the SELECT trial [Lippman 2009] respectively.
The dosage of 285 micrograms of high selenium yeast was used in order to test a dosage of high selenium yeast that contained approximately 200 micrograms of selenomethionine. Speciation studies have shown that high selenium yeast contains up to 70 percent selenomethionine plus numerous other species of selenium [Larsen & Moesgaard 2004].
After nine months of supplementation, the plasma selenium levels had increased by 93% in the l-selenomethionine group, by 54% in the 200-microgram high selenium yeast group, and by 86% in the 285-microgram high selenium yeast group. The plasma selenium levels returned to baseline levels after three months of wash-out.
The research data showed that supplementation with high selenium yeast resulted in significantly reduced levels of bio-markers for oxidative damage. The reduced levels were greatest in individuals with baseline plasma levels of selenium below 127 nanograms per milliliter. Supplementation with l-selenomethionine did not produce significantly reduced levels of bio-markers for oxidative damage.
The study data did not show any changes in serum PSA or blood glucose and glutathione levels.
Given the effectiveness of the high selenium yeast and the ineffectiveness of the l-selenomethionine in lowering bio-markers of oxidative stress, the researchers theorized that it must be selenium species in the high selenium yeast other than the l-selenomethionine in high selenium yeast that causes the decrease in oxidative stress [Richie 2014].
2015: Asemi tests the effect of selenium supplementation on inflammation and oxidative stress in pregnant women with gestational diabetes
Asemi et al carried out a randomized, double-blind, placebo-controlled trial enrolling 70 women with gestational diabetes.
For six weeks starting in the period of the 24th through the 28th week, 35 of the women received 200 micrograms of a high selenium yeast preparation; 35 women received a matching placebo preparation.
The study results showed an association of the selenium supplementation with significant decreases in the serum levels of high-sensitivity C-reactive protein, signifying reduced levels of inflammation in the body, and an association of the selenium supplementation with significant reductions in the plasma levels of malondialdehyde, signifying reduced levels of oxidative stress.
These gains were accompanied by significantly beneficial effects of the supplemental selenium on glucose metabolism in the pregnant women [Asemi 2015].
What have we learned from the selenium and oxidative stress clinical trials?
Selenium supplementation is associated with decreased levels of bio-markers for oxidative stress and is associated with increased levels of antioxidant enzymes. High selenium yeast supplements are more effective against oxidative stress than pure selenomethionine supplements are [Richie 2014].
Selenium and type 2 Diabetes
Observational studies
The results from observational studies regarding the association of selenium intakes and the risk of type 2 diabetes is considered complicated and controversial [Wei 2015, Wang 2016]. Basically, earlier studies dating from 1999 to 2005 seem to show a positive effect of selenium on diabetes [Wei 2015]; later studies dating from 2007 and 2009, seem to indicate that higher levels of selenium intake might be associated with increased prevalence of diabetes [Bleys 2007, Laclaustra 2009]. Meta-analyses done in 2013 and
2015 did not show any significant increase in the risk of diabetes in selenium supplementation groups [Rees 2013, Mao 2015].
2007: Differences between SELECT trial data and NPC trial data
Of the big randomized controlled trials, the SELECT trial did not show any significant association between selenium supplementation and the risk of type 2 diabetes [Lippman 2009]. Follow-up analysis of the data from the Nutritional Prevention of Cancer trial, on the other hand, did seem to show a somewhat increased risk of developing type 2 diabetes in the upper tertile of participants in terms of baseline plasma selenium levels [Stranges 2007].
2008: Cornell study shows absence of indicators of diabetes risk
Combs et al carried out a year-long randomized controlled trial of the dose- response biomarkers of selenium status. The researchers randomly assigned 261 volunteers (106 men and 155 women) to various selenium supplementation groups: 0, 50, 100, or 200 micrograms of selenium in the form of L-selenomethionine daily. The results of the Cornell study did not support a positive association of selenium status and diabetes risk [Combs 2008].
2010: French study shows decreased diabetes risk
In the Epidemiology of Vascular Ageing (EVA) Study, French researchers followed 1162 healthy men and women for nine years. They found that men with the highest blood selenium levels were 50% less likely to have abnormalities in their blood glucose levels that might lead to diabetes than were men with the lowest blood selenium levels. The same significant decreased diabetes risk was not seen in the women in the study. The researchers speculated that the women might have been healthier at the beginning of the study, might have had better antioxidant status, and might possibly process selenium differently from men [Akbaraly 2010].
2012: Harvard study shows lower diabetes risk with higher toenail selenium
A study of selenium levels in the toenails of 7165 men and women done by Harvard and Korean researchers showed that increasing levels of selenium in toenails were associated in a linear relationship with lower risks of type 2 diabetes. The highest levels of toenail selenium content were associated with a 24% reduction in the risk of diabetes [Park 2012].
2012: Rayman investigates possible associations between selenium supplementation and type 2 diabetes
Rayman et al analyzed the data from the UK PRECISE (PREvention of Cancer by Intervention with SElenium) pilot study. The researchers checked plasma adiponectin levels in the participants because plasma adiponectin levels are a recognized independent bio-marker and predictor of type 2 diabetes risk. Adiponectin is a protein that in involved in the regulation of glucose levels and the breakdown of fatty acids.
The UK PRECISE pilot study enrolled 501 elderly British volunteers in a randomized, double-blind, placebo-controlled trial. The volunteers took 100, 200 or 300 micrograms of the SelenoPrecise® high selenium yeast tablets or placebo yeast tablets daily. The mean plasma selenium levels increased significantly in the selenium-treatment groups. The selenium supplementation regimens showed no effect on adiponectin levels during and after six months of treatment.
The researchers concluded that the UK PRECISE data did not show a diabetogenic effect of a six-month supplementation with selenium in elderly individuals with relatively low selenium status at baseline [Rayman 2012].
2013: Rees does a systematic review and meta-analysis showing no significant risk
Rees et al conducted a systematic review and meta-analysis of randomized controlled trials that were selenium only intervention trials reporting the effects of selenium on cardiovascular risk. The researchers included 12 trials enrolling 19,715 participants. The systematic review and meta-analysis showed a small tendency toward increased risk of diabetes, but this small increase was not statistically significant. It could have been due to chance. [Rees 2013].
2015: Chinese study of dietary selenium intakes shows increased risk
A cross-sectional study of selenium intakes and diabetes in middle-aged and elderly Chinese participants showed a significant positive association between dietary selenium intake and diabetes [Wei 2015].
2015: Mao does a meta-analysis of selenium supplementation and risk of type 2 diabetes
Mao et al analyzed four randomized controlled studies that had enrolled 20,294 participants of Caucasian background. The study data did not show that selenium supplementation conferred a significant change, favorable or unfavorable, in the relative risk of developing diabetes [Mao].
2016: Thompson finds age-related difference in incidence of diabetes
Thompson et al in the SEL/CEL trial of selenium supplementation for the prevention of colorectal adenomas found an increased incidence of associated type 2 diabetes in study participants aged 63 or older and reduced incidence in study participants under the age of 63 years [Thompson]. The researchers pointed out that their results can be generalized only to selenium-replete individuals. At baseline, the participants in the SEL/CEL study had a median plasma selenium status of 135 micrograms per liter. By comparison, the participants in the Nutritional Prevention of Cancer study had a baseline plasma selenium status of 114 micrograms per liter, itself a relatively high median compared to baseline values in European and Asian studies.
2016: Wang finds U-shaped relationship between selenium status and risk of diabetes
Wang et al analyzed the data from five observational studies enrolling 13,460 participants. These studies were observational studies rather than experimental studies; therefore, there was no overlap with the studies analyzed by Mao (above).
The researchers found a higher prevalence of type 2 diabetes in the group of participants with the highest blood selenium levels as compared to the group with the lowest selenium levels. In addition, the researchers found a U-shaped relationship between serum selenium levels and prevalence of type 2 diabetes. Serum selenium levels were positively associated with prevalence of type 2 diabetes in participants with relatively low serum selenium levels and relatively high serum selenium levels [Wang 2016].
2016: Mao reports no adverse effect of selenium supplementation of pregnant women on insulin sensitivity and insulin resistance
The Selenium in Pregnancy Intervention (SPRINT) clinical trial enrolled 230 first-time-pregnant women in the UK to take 60 micrograms of high selenium yeast tablets daily in the period from 12 weeks of gestation right through to delivery. The study data showed no adverse effect of the selenium supplementation on plasma adiponectin concentrations in the pregnant women [Mao 2016].
Adiponectin levels are a biomarker for insulin resistance. The researchers found that a nutritional dose of 60 micrograms of high selenium yeast had no adverse effect on the patients’ concentrations of adiponectin in pregnant women with modest selenium status at baseline.
Possible mechanisms relating selenium status to diabetes risk unclear
The mechanisms by which increased selenium intakes might be associated with increased risk of type 2 diabetes in some studies and is not in other studies are unknown. Speculation has focused on possible links between specific selenoproteins and insulin action and glucose metabolism. Nothing definitive has been demonstrated [Rayman 2013].
Steinbrenner, writing in a 2013 review of cell culture and animal studies, thought that selenium intakes from normal diets and from dietary supplements are probably not high enough to induce overt diabetes in healthy individuals. However, he advocated more study of the relationship between selenium and selenoprotein activity, on the one hand, and carbohydrate and lipid metabolism, on the other hand.
Selenium and Pregnancy
Observational studies
There has not yet been adequate clinical research done on the beneficial health effects of selenium supplementation in pregnant women; however, a start has been made, as can be seen in the clinical studies summarized below. Observational studies indicate that selenium deficiencies may cause complications during pregnancy including miscarriages and possible damaging of the nervous and immune systems of the fetus. Low selenium status in the early stages of pregnancy has been associated with low birth weight in newborns [Pieczyńska 2015]. There is a need for validation of the study results presented below in adequately powered trials.
1994: Han reports that selenium supplementation prevents pregnancy-induced hypertension
Han et al carried out a randomized controlled trial in which 52 pregnant women received 100 micrograms of a liquid dietetic selenium preparation daily and 48 pregnant women received a matching placebo. The results showed that the selenium dietary supplement prevented and decreased the incidence of pregnancy-induced hypertension and gestational edema. The selenium supplementation did not affect the neonatal birth weight or the amount of postpartum hemorrhage [Han 1994].
2007: Negro reports the influence of selenium supplementation on postpartum thyroid status
Negro et al did a randomized controlled trial to see if selenium supplementation during and after pregnancy influences thyroid function. They randomly assigned 77 pregnant women who were positive for thyroid peroxidase autoantibodies to an active treatment group receiving 200 micrograms of a selenomethionine preparation daily and 74 pregnant women who were positive for thyroid peroxidase autoantibodies to a placebo group. They also selected 81 age-matched women for a control group.
The researchers’ measurements showed that the incidence of postpartum thyroid dysfunction and, thus, the risk of permanent hypothyroidism were significantly lower in the selenium supplementation group than in the placebo group. They concluded that supplementation with selenium reduced thyroid inflammatory activity and the incidence of hypothyroidism [Negro 2007].
2010: Tara reports the effect of selenium supplementation on oxidative stress, preeclampsia, and pre-labor rupture of membranes
Tara et al randomly assigned 166 first-time-pregnant women in their first trimester to an active treatment group receiving 100 micrograms of SelenoPrecise® high selenium yeast or to a placebo control group. They evaluated the effect of the supplementation in three distinct areas and published the following research findings [Tara 2010]:
- Selenium supplementation reduces the extent of oxidative stress associated with pregnancy.
- Selenium supplementation of pregnancy women reduces the frequency of preeclampsia during pregnancy.
- Supplementation with selenium significantly reduces the incidence of premature (pre-labor) rupture of membranes in pregnant women.
2011: Mokhber reports the effect of selenium supplementation on postpartum depression
Mokhber et al used data from the same sample of pregnant women in the Tara research studies (above) in which 166 first-time-pregnant women in their first trimester were randomly assigned to an active treatment group receiving 100 micrograms of SelenoPrecise® high selenium yeast or to a placebo control group. Eight weeks after delivery, the researchers used the Edinburgh Postnatal Depression Scale to evaluate the symptoms of postpartum depression.
The mean depression scale scores were significantly lower in the selenium treatment group than they were in the placebo group. Supplementation with selenium might be an effective way to prevent postpartum depression [Mokhber 2011].
2012: Boskabadi reports the effect of prenatal selenium supplementation on cord blood selenium and lipid profile
Boskabadi et al also used data from the 166 first-time-pregnant women who were randomly assigned to an active selenium treatment group or to a placebo control group. The women in the active treatment group received 100 micrograms of the SelenoPrecise® high selenium yeast daily. The researchers did not observe any significant differences in cord blood selenium levels or in cord blood cholesterol levels. Triglyceride levels were significantly higher in the selenium treatment group. The clinical significance of this finding needs to be evaluated [Boskabadi 2012].
2014-2016: Rayman reports the results of the Selenium in Pregnancy Intervention (SPRINT) trial
Much of what we know about selenium supplementation in pregnancy has come from the data of the Selenium in Pregnancy Intervention (SPRINT) trial, a randomized controlled trial carried out in the United Kingdom, which is a region of the world in which women are likely to have low selenium status. The selenium used in the SPRINT trial was the SelenoPrecise® high selenium yeast formulation.
Starting in the women’s 12th week of pregnancy and continuing to the date of childbirth, 230 first-time-pregnant women were randomly assigned to an active treatment group receiving 60 micrograms of selenium daily or to a placebo control group. To date, the researchers have reported the following results:
- There was no adverse effect on a recognized bio-marker – plasma adiponectin concentration – for insulin resistance in the pregnant women taking the selenium supplementation. The researchers regarded the lack of any adverse effect as reassuring, given that there has been concern in some quarters about a possible association between selenium status and type 2 diabetes [Mao 2016].
- Compared with the placebo supplement, the selenium supplement significantly reduced the pregnant women’s odds ratio for the development of pregnancy-induced hypertension [Rayman 2015].
- The selenium supplementation of the pregnant women was significantly associated with a reduction in serum soluble vascular endothelial growth factor receptor-1 (abbreviated sFlt-1), which is an anti-angiogenic factor known to be related to the risk of pre-eclampsia. Pre-eclampsia is a serious pregnancy complication characterized by high blood pressure that can lead to serious and even fatal outcomes for both the mother and the fetus [Rayman 2014].
- The selenium supplementation in pregnant women had no effect on thyroid peroxidase antibody concentrations. Measuring thyroid peroxidase antibody levels is a good test for the presence of autoimmune thyroid diseases such as Hashimoto thyroiditis and Graves’ disease. The researchers did note a non-significant tendency for the selenium supplementation to change thyroid function in women who were positive at baseline with respect to thyroid peroxidase antibody levels [Mao 2016].
- The SPRINT researchers also found significant associations between whole blood selenium concentrations and genetic variation in the coding for the enzyme dimethylglycine dehydrogenase (DMGDH). The study results also showed that women with the SEPP1 rs3877899 A allele maintain selenium status better during pregnancy, and, moreover, they show increased GPx3 enzyme activity with selenium supplementation [Mao 2016].
2015: Asemi reports on the effects of selenium supplementation on glucose metabolism, inflammation, and oxidative stress in pregnant women
Asemi et al tested the effects of daily selenium supplementation on pregnant women with gestational diabetes who were not taking any oral hypoglycemic medicine. The researchers randomly assigned 70 women with gestational diabetes to either a 200-microgram selenium treatment group or to a placebo group for 6 weeks starting in one of the weeks between week 24 and week 28 of pregnancy. Compared with the placebo treatment, the selenium supplementation resulted in several significant outcomes:
- reduction in fasting plasma glucose, serum insulin levels, and insulin resistance
- increase in insulin sensitivity
- decrease in a serum high-sensitivity C-reactive protein (a bio-marker for inflammation)
- elevation of plasma glutathione
- reduction of plasma malondialdehyde levels (a bio-marker for oxidative stress)
Moreover, there was no alteration of lipid profiles, plasma nitric oxide, or total antioxidant
capacity concentrations in the pregnant women who received the selenium
supplementation. The selenium supplementation was associated with beneficial effects on glucose metabolism, inflammation, and oxidative stress in pregnant women with gestational diabetes [Asemi 2015].
2016: Xu does a review of selenium and pre-eclampsia studies
Xu et al did a systematic review and meta-analysis of the research literature relating selenium and pre-eclampsia. They reviewed 13 observational studies with 1515 participants and 3 randomized controlled trials with 439 participants.
Based on the results of the randomized controlled trials, women taking a selenium supplement had 72% less risk of contracting pre-eclampsia compared to women taking the placebo supplement. The available study results show an inverse association of blood selenium level and the risk of preeclampsia. Selenium supplementation significantly reduces the incidence of preeclampsia. The authors recommend further studies to gather more information about the most effective dosage, the best beginning time during pregnancy, and the optimal duration of the selenium supplementation.
What have we learned from the selenium in pregnancy clinical trials?
Selenium supplementation of pregnant women during pregnancy is associated with several health benefits:
- reduced incidence of pregnancy-induced hypertension
- reduced bio-markers of oxidative stress
- reduced incidence of pre-eclampsia
- reduced incidence of premature (pre-labor) rupture of membranes
- reduced incidence of postpartum depression
- no adverse effects related to insulin sensitivity and insulin resistance
Selenium and the Elderly
Cardoso et al measured selenium status in the plasma and in the red blood cells of elderly individuals diagnosed with Alzheimer’s disease or with mild cognitive impairment. They compared the selenium status measurements with the selenium status data from a control group of elderly individuals [Cardoso 2014]
Their study data showed the lowest plasma selenium levels in the Alzheimer’s disease group. The data also showed that the red blood cell selenium concentrations in the Alzheimer’s disease group were significantly lower than the red blood cell concentrations in the control group but not significantly lower than the red blood cell concentrations in the group of individuals with mild cognitive impairment.
The researchers concluded that red blood cell selenium concentrations decline as cognitive function declines. The significance of this finding is that red blood cell levels reflect longer-term selenium status; consequently, there seems to be a relationship between selenium exposure and cognitive function. The researchers concluded that low selenium status in the elderly most likely contributes to cognitive decline [Cardoso 2014].
1988: Tolonen reports on antioxidant supplementation including selenium in the elderly
Tolonen et al enrolled 45 elderly Finnish nursing home residents in a 12-month matched pairs randomized, double-blind, placebo-controlled study. There were 31 women and 14 men, average age 80.5 years (range 66 – 96 years) in the study.
The nursing home residents who received the daily antioxidant treatment received the following active ingredients:
- 100 micrograms of selenium as l-selenomethionine (Bio-Selenium)
- 15 milligrams of zinc
- 9 milligrams of beta-carotene
- 90 milligrams of Vitamin C
- 400 mg of Vitamin E
The study showed the following beneficial results of the antioxidant supplementation:
- Reduced lipid peroxide levels, measured by assays of thiobarbituric acid reactive substances (tbars are the byproducts of lipid peroxidation); there was no corresponding decrease in the tbars levels of the placebo group
- A significant inverse relationship between the concentration levels of serum tbars reactants and whole blood selenium; the decrease in serum lipid peroxides was noted only when whole blood selenium reached levels greater than 200 micrograms/liter
- A slight but encouraging improvement on several psychological tests; there was no corresponding improvement in the placebo group
- Reports by the nursing staff of apparent clinical improvement in the treatment group participants
- No toxic side effects reported [Tolonen 1988]

Illustration: Professor Jørgen Clausen (Denmark, left) and Professor Matti Tolonen (Finland, center) worked closely with Sven Moesgaard (right) in the testing of various formulations of selenium supplements and in the testing of the antioxidant supplements in elderly populations.
1988: Westermarck reports statistically significant health outcomes in the elderly
Westermarck et all carried out a 12-month randomized, double-blind, placebo-controlled study of the effects of an antioxidant combination on 70 elderly people living in a nursing home, average age 80 years.
The daily dosages used were as follows:
- Selenium: 100 micrograms of organic selenomethionine (Bio-Selenium) and 800 micrograms of inorganic sodium selenate
- Beta-carotene: 9 milligrams
- Vitamin B-6: 2 milligrams
- Vitamin C: 90 milligrams
- Vitamin E: 460 milligrams
- Zinc: 15 milligrams
The study data showed the following statistically significant outcomes in the active treatment group as compared with the placebo group:
- Increased levels of selenium in plasma
- Increased levels of glutathione peroxidase antioxidant enzyme activity
- Decreased lipid peroxidation measured in terms of malondialdehyde levels
- Improvement on several parameters of psychological tests
- Clinical improvement of patients
- No toxic side effects
1989: Clausen reports on the clinical effects of an antioxidative supplementation of elderly individuals
Clausen recruited 97 geriatric patients (average age: 80 years) from two Danish assisted living facilities to participate in a randomized, double-blind, placebo-controlled study. The year-long study tested the effects of an antioxidative cocktail on ageing phenomena.
The patients in the active treatment group received the following ingredients daily:
- 300 micrograms selenium as L-selenomethionine (Bio-Selenium)
- 45 milligrams of zinc
- 2.7 milligrams of vitamin A
- 6 milligrams of vitamin B-6
- 270 milligrams of vitamin C
- 465 milligrams of vitamin E (d-alpha-tocopherol)
- 250 milligrams of gamma-linolenic acid
The patients in the placebo group received matching tablets and capsules without the active components.
The study data showed that the selenium supplementation raised the levels of the selenium in whole blood, the levels of glutathione peroxidase in the red blood cells, and the levels of vitamin E level in serum, compared to baseline levels and to the corresponding levels in the placebo group.
The researchers measured the changes in the lipofuscin levels in the elderly patients. Lipofuscin is the build-up of the granular age-related pigments found in the adrenal glands, heart, kidney, liver, nerve cells, and retina. The presence of these granules is thought to be a sign of ageing and of wear and tear on the body.
In the group treated with the antioxidant cocktail that included 300 micrograms of selenium, the lipofuscin level declined significantly compared to the placebo group.
In addition, during the year of supplementation, the researchers noted slight but significant improvements in the psychological scores of the patients in the antioxidant cocktail group. The researchers’ assays of blood flow in different areas of the brain surface showed generally improved blood flow in the selenium group when compared with baseline values and when compared with the values from the placebo group [Clausen 1989].

Illustration: Professor Jørgen Clausen taught and did research in the Institute for Life Sciences and Chemistry, University of Roskilde, Denmark. His research interests included the study of selenium in chronic neurologic diseases and in the elderly as well as the comparison of whole blood selenium values and red blood cell glutathione peroxidase activity in normal individuals supplemented with various forms of selenium: selenate, selenite, L-selenomethionine, and high selenium yeast.
2013-2016: Alehagen reports on the effects of combined selenium and Coenzyme Q10 supplementation in healthy elderly citizens (the KiSel-10 study)
Daily supplementation of elderly Swedish citizens for five years with 200 micrograms of
SelenoPrecise® high selenium yeast and with 200 milligrams of Bio-Quinone Q10 Gold Coenzyme Q10 or similar placebos significantly lowered the risk of dying from cardiovascular disease by 54% [Alehagen 2013]. Measurements of the heart function of the study participants in the active treatment group showed significant improvement compared to the participants in the placebo group.
What have we learned from the selenium and the elderly clinical trials?
Supplementation with selenium and Coenzyme Q10 reduces cardiovascular mortality, reduces the levels of bio-markers for inflammation and oxidative stress, and reduces the number and duration of hospital visits. Supplementation with selenium and Coenzyme Q10 improves the quality of life of senior citizens. Supplementation with selenium may slow the pace of cognitive decline.
Illustration: SelenoPrecise® tablets as selenium yeast and Bio-Quinone CoQ10 Softgels are both pharmaceutical grade preparations used in numerous scientific studies.
When the participants in the KiSel-10 study were grouped for analysis by age, gender, and heart function status at baseline, the researchers saw that more participants in the placebo group had to be admitted to the hospital during the study than participants in the combined high selenium yeast and Coenzyme Q10 group. The participants in the active treatment group had better quality of life, better physical performance, and better cognitive functioning than participants in the placebo group [Johansson 2015].
Moreover, selenium supplementation was associated with significant improvement in a biomarker for heart disease, the NT-proBNP peptide [Johansson 2013]. The supplementation with the high selenium yeast and Coenzyme Q10 was positively associated with reductions in biomarkers for inflammation and oxidative stress in the elderly participants [Alehagen 2015].
Selenium and Smokers
Observational studies
Schöpfer and Schrauzer have observed that higher exposures of cadmium, especially in smokers, may lead to the weakening or negating of the anti-carcinogenic effects of selenium and may increase prostate cancer risk in men as they increase in age. When the accumulating levels of cadmium exceed the levels of the available neutralizing selenium in the body, the detoxification of the cadmium is less and less efficient, particularly in the prostate [Schöpfer 2010].
1992: Clausen reports that selenium supplementation may decrease the level of oxidative stress associated with smoking
Clausen et al did studies that showed that the extent of respiratory burst reactions of neutrophils in the peripheral blood is significantly higher in smokers than in non-smokers. The respiratory burst reactions are the release of harmful free radicals, in particular superoxide radicals and hydrogen peroxide radicals, which are the cause of increased oxidative stress.
Clausen supplemented smokers’ diets with the following antioxidants for ten days:
- 200 micrograms of selenium as L-Selenomethionine (Bio-Selenium*)
- 1000 milligrams of vitamin E
* Later changed to selenium yeast
After just ten days of selenium and vitamin E supplementation, the respiratory burst reactions of the smokers had significantly decreased by 20-75 percent. Clausen concluded that it may be necessary for smokers to use relatively high doses of selenium in order to attenuate the pathological processes associated with smoking.
Summary and Conclusion
Summary
Selenium is an essential nutrient. The intakes of selenium and the selenium status of individuals vary widely depending on which region of the world they live in and what sort of diet they eat.
The intake in Europe is according to statistics very low, and in some parts of China supplementation is an absolute need. The need for selenium supplementation in the United States is seemingly higher along the coasts than it is in the middle of the country. The range of optimal intake is relatively narrow, but positive benefits has been shown in studies with total intake more than 150 micrograms per day up to 300 micrograms per day, which is generally regarded as safe and beneficial to health. There is a recommendation not to exceed 400 micrograms of total intake per day.
Selenium is especially important for its bio-availability as a co-factor in the production of the glutathione peroxidase antioxidant enzymes. In addition, it is necessary for the production of some 25 selenoproteins, which have various functions in the body, not all of which have been charted as yet.
Among the important functions of selenium are the following activities to which selenium in one form or another contributes (not listed in rank order):
- Antioxidant function, preventing harmful free radicals from damaging cells and DNA
- Anti-carcinogenic function
- Cognitive functioning
- Detoxification of heavy metals
- Immune system response function
- Muscle strength function, especially heart muscle strength and function
- Slowing of the progression of HIV/AIDS
- Sperm production function
- Thyroid hormone regulation function
The European Food Safety Agency EFSA acknowledges selenium's importance for our immune system, thyroid function, sperm production, growth of hair and nails as well as its role in protection against oxidative stress.
Conclusion
At this writing (Winter, 2016), cell culture studies and animal studies and human observational studies provide good arguments for the use of selenium supplementation in populations that live in selenium-poor regions of the world.
More randomized, double-blind, placebo-controlled clinical trials are needed.
Unfortunately, it is difficult to get funding for big and expensive gold standard studies because selenium preparations cannot be patented.
The present state of the clinical trial literature shows the following picture:
Cancer
The evidence from animal studies and human observational studies supports the assertion of a strong association between selenium status and risk of cancer. The clinical trial picture has been blurred somewhat by the different outcomes of the Nutritional Prevention of Cancer trial and the Selenium and Vitamin E Cancer Prevention Trial (SELECT trial). At least three large randomized controlled trials with a high selenium yeast preparation have shown significant reductions in the risk of cancer [Blot, Clerk, Hercberg]. What is needed is funding for a revival of The Prevention of Cancer by Intervention with Selenium (PRECISE) clinical trial, which was halted after the pilot study stage because of a lack of funding. The preparation used in the PRECISE pilot studies was the SelenoPrecise® high selenium yeast preparation.
Cardiovascular disease
Animal studies and observational studies provide evidence that selenium deficiency is associated with loss of muscle strength, including heart muscle strength, and with increased inflammation. The results from the KiSel-10 clinical trial of combined selenium and Coenzyme Q10 supplementation of healthy elderly citizens showed significantly reduced cardiovascular mortality, inflammation, and oxidative damage.
Thyroid function
Supplementation with selenium in selenium-deficient (and iodine-deficient) individuals has been linked to improved thyroid metabolism, improved synthesis of thyroid hormones, and reduction of oxidative stress in the thyroid. In particular, the beneficial effect of selenium supplementation has been shown in autoimmune thyroid disorders. Selenium supplementation improves thyroid function in patients with Hashimoto’s disease and facilitates the return to normal thyroid functioning in patients with Graves’ disease.
HIV/AIDS
Observational studies show that selenium status is low in HIV-infected individuals and declines with the progression of the infection to AIDS. There is some but not enough clinical trial evidence that selenium supplementation provides a significant clinical benefit to patients on antiretroviral medications. For HIV-infected patients not on anti-retroviral medications, supplementation with selenium and other micronutrients has been showed to be safe and effective in slowing the progression of the disease and effective in reducing the risk of immune system decline and death. To be effective, the supplementation with selenium and micronutrients must be started in the early stages of the HIV infection.
Asthma
Small clinical trials have shown that selenium supplementation of bronchial asthma patients is associated with reduced oxidative stress and reduced chronic inflammation. More clinical trials are warranted.
Pregnancy
Small but well-designed clinical trials have shown that selenium supplementation during pregnancy is associated with reduced oxidative stress, reduced incidence of pre-eclampsia, reduced incidence of premature (pre-labor) rupture of membranes, and reduced incidence of postpartum depression.
Elderly
The results of the KiSel-10 clinical trial and of earlier smaller clinical trials show that selenium supplementation, combined with Coenzyme Q10 supplementation, reduces cardiovascular mortality, the levels of bio-markers for inflammation and oxidative stress, and the number and duration of hospital visits. Clinical trial evidence shows that supplementation with selenium and Coenzyme Q10 improves the quality of life of senior citizens.
Form and dosage
At present, the evidence from speciation and bio-availability studies and clinical trials indicates that high selenium yeast (also known as selenized yeast or selenium-enriched yeast) preparations provide the widest range of selenium species. This is important because selenium is used in many different activities and functions in the body, and different selenium compounds seem to have different effects.
Proper dosage needs to be studied in relation to region and diet. Selenium dosage also needs to be studied in relation to the associated effect on selenium containing antioxidant enzymes and in relation to selenium’s chemopreventive effects on cancer.
Given the present state of knowledge about selenium supplementation, the following rules of thumb would seem to apply, again recognizing regional differences in diet:
- A daily selenium supplement of 100 to 200 micrograms is safe.
- Total daily selenium intake from all sources should not exceed 400 micrograms (FDA).
- Taking a daily combination of selenium and Coenzyme Q10 supplements gives greater effect than taking either supplement alone.
Sources
Akbaraly, T. N., Arnaud, J., Rayman, M. P., Hininger-Favier, I., Roussel, A., Berr, C., & Fontbonne, A. (2010). Plasma selenium and risk of dysglycemia in an elderly French population: results from the prospective Epidemiology of Vascular Ageing (EVA) Study. Nutrition & Metabolism, 721.
Akesson, I., Schutz, A., Attewell, R., Skerfving, S. & Glantz, P.O. (1991). Status of mercury and selenium in dental personnel: impact of amalgam work and own fillings. Arch Environ Health, 46(2):102-9.
Alatise, O. I., & Schrauzer, G. N. (2010). Lead exposure: a contributing cause of the current breast cancer epidemic in Nigerian women. Biological Trace Element Research, 136(2), 127-139.
Alehagen, U., Johansson, P., Björnstedt, M., Rosén, A., & Dahlström, U. (2013). Cardiovascular mortality and N-terminal-proBNP reduced after combined selenium and coenzyme Q10 supplementation: a 5-year prospective randomized double-blind placebo-controlled trial among elderly Swedish citizens. International Journal of Cardiology, 167(5), 1860-1866.
Alehagen, U., Lindahl, T. L., Aaseth, J., Svensson, E., & Johansson, P. (2015). Levels of sP-selectin and hs-CRP decrease with dietary intervention with selenium and Coenzyme Q10 combined: A Secondary analysis of a randomized clinical trial. Plos ONE, 10(9), 1-16.
Alehagen, U., Aaseth, J., & Johansson, P. (2015). Reduced Cardiovascular Mortality 10 Years after Supplementation with Selenium and Coenzyme Q10 for Four Years: Follow-Up Results of a Prospective Randomized Double-Blind Placebo-Controlled Trial in Elderly Citizens. Plos One, 10(12), e0141641.
Alehagen, U., Aaseth, J., & Johansson, P. (2015). Less increase of copeptin and MR- proADM due to intervention with selenium and coenzyme Q10 combined: Results from a 4-year prospective randomized double-blind placebo-controlled trial among elderly Swedish citizens. Biofactors (Oxford, England), 41(6), 443-452.
Alehagen, U., & Aaseth, J. (2015). Selenium and coenzyme Q10 interrelationship in cardiovascular diseases--A clinician's point of view. Journal of Trace Elements in Medicine and Biology, 31157-162.
Alehagen U., Johansson P., Bjornstedt M., & Rosen A. (2016). Relatively high mortality risk in elderly Swedish subjects with low selenium status. European Journal of Clinical Nutrition, 70(1): 91-96.
Amoako, P. O., Uden, P. C., & Tyson, J. F. (2009). Speciation of selenium dietary supplements; formation of S- (methylseleno)cysteine and other selenium compounds. Analytica Chimica Acta, 652(1-2), 315-323.
Algotar, A. M., Stratton, M. S., Ahmann, F. R., Ranger-Moore, J., Nagle, R. B., Thompson, P. A., & Stratton, S. P. (2013). Phase 3 clinical trial investigating the effect of selenium supplementation in men at high-risk for prostate cancer. The Prostate, 73(3), 328-335.
Arber, N., Spicak, J., Rácz, I., Zavoral, M., Breazna, A., Gerletti, P., & ... Levin, B. (2011). Five-year analysis of the prevention of colorectal sporadic adenomatous polyps trial. The American Journal of Gastroenterology, 106(6), 1135-1146.
Asemi, Z., Jamilian, M., Mesdaghinia, E., & Esmaillzadeh, A. (2015). Effects of selenium supplementation on glucose homeostasis, inflammation, and oxidative stress in gestational diabetes: Randomized, double-blind, placebo-controlled trial. Nutrition (Burbank, Los Angeles County, Calif.), 31(10), 1235-1242.
Assmann, K. E., Andreeva, V. A., Jeandel, C., Hercberg, S., Galan, P., & Kesse-Guyot, E. (2015). Healthy Aging 5 Years After a Period of Daily Supplementation With Antioxidant Nutrients: A Post Hoc Analysis of the French Randomized Trial SU.VI.MAX. American Journal of Epidemiology, 182(8), 694-704.
Babaknejad, N., Sayehmiri, F., Sayehmiri, K., Rahimifar, P., Bahrami, S., Delpesheh, A., & Alizadeh, S. (2014). The relationship between selenium levels and breast cancer: a systematic review and meta-analysis. Biological Trace Element Research, 159(1-3), 1-7.
Bates, M. N. (2006). Mercury amalgam dental fillings: an epidemiologic assessment. International Journal of Hygiene and Environmental Health, 209(4), 309-316.
Baum, M. K., Campa, A., Lai, S., Sales Martinez, S., Tsalaile, L., Burns, P., & Marlink, R. (2013). Effect of micronutrient supplementation on disease progression in asymptomatic, antiretroviral-naive, HIV-infected adults in Botswana: a randomized clinical trial. Jama, 310(20), 2154-2163.
Bekaert B., Cooper M.L., Green F.R., McNulty H., Pentieva K., Scott J.M., Molloy A.M., & Rayman M.P. (2008). Effect of selenium status and supplementation with high-selenium yeast on plasma homocysteine and B vitamin concentrations in the UK elderly, Mol Nutr Food Res, 52, 11, 1324-1333.
Bellinger, F. P., Raman, A. V., Reeves, M. A., & Berry, M. J. (2009). Regulation and function of selenoproteins in human disease. The Biochemical Journal, 422(1), 11- 22.
Berry, M. J. & Ralston, N. C. (2008). Mercury toxicity and the mitigating role of selenium. Ecohealth, 5(4), 456-459.
Bleys J., Navas-Acien A., & Guallar E. (2007). Serum selenium and diabetes in U.S. adults. Diabetes Care, 30(4):829-834.
Bleys, J., Navas-Acien, A., & Guallar, E. (2008). Serum selenium levels and all-cause, cancer, and cardiovascular mortality among US adults. Archives of Internal Medicine, 168(4), 404-410.
Blot, W. J., Li, J. Y., Taylor, P. R., Guo, W., Dawsey, S., Wang, G. Q., & Li, B. (1993). Nutrition intervention trials in Linxian, China: supplementation with specific vitamin/mineral combinations, cancer incidence, and disease-specific mortality in the general population. Journal of The National Cancer Institute, 85(18), 1483- 1492.
Bogsrud, M. P., Langslet, G., Ose, L., Arnesen, K., Sm Stuen, M. C., Malt, U. F., & Retterstøl, K. (2013). No effect of combined coenzyme Q10 and selenium supplementation on atorvastatin-induced myopathy. Scandinavian Cardiovascular Journal: SCJ, 47(2), 80-87.
Bonelli, L., Puntoni, M., Gatteschi, B., Massa, P., Missale, G., Munizzi, F., & Bruzzi, P. (2013). Antioxidant supplement and long-term reduction of recurrent adenomas of the large bowel. A double-blind randomized trial. Journal of Gastroenterology, 48(6), 698-705.
Boskabadi, H., Maamouri, G., Rezagholizade Omran, F., Mafinejad, S., Tara, F., Rayman, M. P., & Ferns, G. A. (2012). Effect of prenatal selenium supplementation on cord blood selenium and lipid profile. Pediatrics and Neonatology, 53(6), 334- 339.
Bügel, S., Larsen, E. H., Sloth, J. J., Flytlie, K., Overvad, K., Steenberg, L. C., & Moesgaard, S. (2008). Absorption, excretion, and retention of selenium from a high selenium yeast in men with a high intake of selenium. Food & Nutrition Research, 52.
Bülow Pedersen, I., Knudsen, N., Carlé, A., Schomburg, L., Köhrle, J., Jørgensen, T., & Laurberg, P. (2013). Serum selenium is low in newly diagnosed Graves' disease: a population-based study. Clinical Endocrinology, 79(4), 584-590.
Burbano, X., Miguez-Burbano, M. J., McCollister, K., Zhang, G., Rodriguez, A., Ruiz, P., & Shor-Posner, G. (2002). Impact of a selenium chemoprevention clinical trial on hospital admissions of HIV-infected participants. HIV Clinical Trials, 3(6), 483- 491.
Cai, X., Wang, C., Yu, W., Fan, W., Wang, S., Shen, N., & ... Wang, F. (2016). Selenium Exposure and Cancer Risk: an Updated Meta-analysis and Meta- regression. Scientific Reports, 619213.
Cardoso, B. R., Silva Bandeira, V., Jacob-Filho, W., & Franciscato Cozzolino, S. M. (2014). Selenium status in elderly: relation to cognitive decline. Journal of Trace Elements in Medicine and Biology, 28(4), 422-426.
Cardoso, B. R., Roberts, B. R., Bush, A. I. & Hare, D. J. (2015). Selenium, selenoproteins, and neurodegenerative diseases. Metallomics, 7, 1213-1225.
Clark, L. C., Combs, G. J., Turnbull, B. W., Slate, E. H., Chalker, D. K., Chow, J., & Taylor, J. R. (1996). Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin. A randomized controlled trial. Nutritional Prevention of Cancer Study Group. Jama, 276(24), 1957-1963.
Clark, L. C., Dalkin, B., Krongrad, A., Combs, G. J., Turnbull, B. W., Slate, E. H., & Rounder, J. (1998). Decreased incidence of prostate cancer with selenium supplementation: results of a double-blind cancer prevention trial. British Journal of Urology, 81(5), 730-734.
Clausen, J., & Nielsen, S. A. (1988). Comparison of whole blood selenium values and erythrocyte glutathione peroxidase activities of normal individuals on supplementation with selenate, selenite, L-selenomethionine, and high selenium yeast. Biological Trace Element Research, 15125-138.
Clausen, J., Jensen, G. E., & Nielsen, S. A. (1988). Selenium in chronic neurologic diseases. Multiple sclerosis and Batten's disease. Biological Trace Element Research, 15179-203.
Clausen, J., Nielsen, S. A., & Kristensen, M. (1989). Biochemical and clinical effects of an antioxidative supplementation of geriatric patients. A double blind study. Biological Trace Element Research, 20(1-2), 135-151.
Clausen, J. (1992). The influence of antioxidants on the enhanced respiratory burst reaction in smokers. Annals of The New York Academy Of Sciences, 669, 337-341.
Clayton, C. C. & Baumann, C. A. (1949). Diet and azo dye tumors; effect of diet during a period when the dye is not fed. Cancer Research, 9(10):575-82.
Cold, F., Winther, K.H., Pastor-Barriuso, R., Rayman, M.P., Guallar, E., Nybo, M., Griffin, B.A., Stranges, S., & Cold, S. (2015). Randomised controlled trial of the effect of long-term selenium supplementation on plasma cholesterol in an elderly Danish population. British Journal of Nutrition, 114(11):1807-18.
Combs, G. F., Combs, S. B. (1998). Chemopreventive agents: selenium. Pharmacology & Therapeutics, 79(3), 179.
Combs, G. F. (2001). Impact of selenium and cancer-prevention findings on the nutrition-health paradigm. Nutrition and Cancer, 40(1), 6-11.
Combs, G. F. (2005). Current evidence and research needs to support a health claim for selenium and cancer prevention. The Journal of Nutrition, 135(2), 343-347.
Combs, G.F., Watts, J.J., Johnson,K., Canfield, W.K., Davis, C.D. & Milner, J.A. (2008). Absence of diabetes indicators in a selenium-supplementation trial. J. Fed. Amer. Socs. Expl Biol. 22: Abstract 696.4.
De Waal, H., Stam, C. J., Lansbergen, M. M., Wieggers, R. L., Kamphuis, P. H., Scheltens, P., & van Straaten, E. W. (2014). The effect of Souvenaid on functional brain network organisation in patients with mild Alzheimer's disease: a randomised controlled study. Plos One, 9(1), e86558.
Drutel, A., Archambeaud, F., & Caron, P. (2013). Selenium and the thyroid gland: more good news for clinicians. Clinical Endocrinology, 78(2), 155-164.
El-Bayoumy, K., Richie, J. J., Boyiri, T., Komninou, D., Prokopczyk, B., Trushin, N., & Colosimo, S. (2002). Influence of selenium-enriched yeast supplementation on biomarkers of oxidative damage and hormone status in healthy adult males: a clinical pilot study. Cancer Epidemiology, Biomarkers & Prevention, 11(11), 1459- 1465.
Etminan, M., FitzGerald, J. M., Gleave, M., & Chambers, K. (2005). Intake of selenium in the prevention of prostate cancer: a systematic review and meta- analysis. Cancer Causes & Control: CCC, 16(9), 1125-1131.
Fedacko, J., Pella, D., Fedackova, P., Hänninen, O., Tuomainen, P., Jarcuska, P., & Littarru, G. P. (2013). Coenzyme Q10 and selenium in statin-associated myopathy treatment. Canadian Journal of Physiology and Pharmacology, 91(2), 165-170.
Galan, P., Briançon, S., Favier, A., Bertrais, S., Preziosi, P., Faure, H., & Hercberg, S. (2005). Antioxidant status and risk of cancer in the SU.VI.MAX study: is the effect of supplementation dependent on baseline levels? The British Journal of Nutrition, 94(1), 125-132.
Geybels, M. S., Verhage, B. J., van Schooten, F. J., Goldbohm, R. A., & van den Brandt, P. A. (2013). Advanced prostate cancer risk in relation to toenail selenium levels. Journal of The National Cancer Institute, 105(18), 1394-1401.
Goossens, M. E., Buntinx, F., Joniau, S., Ackaert, K., Ameye, F., Billiet, I., & Zeegers, M. P. (2012). Designing the selenium and bladder cancer trial (SELEBLAT), a phase lll randomized chemoprevention study with selenium on recurrence of bladder cancer in Belgium. BMC Urology, 128.
Han, L., & Zhou, S. M. (1994). Selenium supplement in the prevention of pregnancy induced hypertension. Chinese Medical Journal, 107(11), 870-871.
Hatfield, D. L., & Gladyshev, V. N. (2009). The Outcome of Selenium and Vitamin E Cancer Prevention Trial (SELECT) reveals the need for better understanding of selenium biology. Molecular Interventions, 9(1), 18-21.
Haug, A., Graham, R. D., Christophersen, O. A., & Lyons, G. H. (2007). How to use the world's scarce selenium resources efficiently to increase the selenium concentration in food. Microbial Ecology in Health and Disease, 19(4), 209-228.
Helzlsouer, K. J., Huang, H. Y., Alberg, A. J., Hoffman, S., Burke, A., Norkus, E. P., & Comstock, G. W. (2000). Association between alpha-tocopherol, gamma- tocopherol, selenium, and subsequent prostate cancer. Journal of The National Cancer Institute, 92(24), 2018-2023.
Hercberg, S., Galan, P., Preziosi, P., Bertrais, S., Mennen, L., Malvy, D., & Briançon, S. (2004). The SU.VI.MAX Study: a randomized, placebo-controlled trial of the health effects of antioxidant vitamins and minerals. Archives of Internal Medicine, 164(21), 2335-2342.
Hernaman-Johnson, F. (1935). Selenium in the treatment of cancer. British Medical Journal, 1(3877): 1052-1053.
Hurst, R., Armah, C. N., Dainty, J. R., Hart, D. J., Teucher, B., Goldson, A. J., & Fairweather-Tait, S. J. (2010). Establishing optimal selenium status: results of a randomized, double-blind, placebo-controlled trial. The American Journal of Clinical Nutrition, 91(4), 923-931.
Hurwitz, B. E., Klaus, J. R., Llabre, M. M., Gonzalez, A., Lawrence, P. J., Maher, K. J., & Schneiderman, N. (2007). Suppression of human immunodeficiency virus type 1 viral load with selenium supplementation: a randomized controlled trial. Archives of Internal Medicine, 167(2), 148-154.
Hussein, O., Rosenblat, M., Refael, G., & Aviram, M. (1997). Dietary selenium increases cellular glutathione peroxidase activity and reduces the enhanced susceptibility to lipid peroxidation of plasma and low-density lipoprotein in kidney transplant recipients. Transplantation, 63(5), 679-685.
Jackson, M. I., & Combs, G. J. (2008). Selenium and anti-carcinogenesis: underlying mechanisms. Current Opinion in Clinical Nutrition and Metabolic Care, 11(6), 718-726.
Jensen, G. E., & Clausen, J. (1983). Leucocyte glutathione peroxidase activity and selenium level in Batten's disease. Scandinavian Journal of Clinical and Laboratory Investigation, 43(3), 187-196.
Johansson, P., Dahlström, Ö., Dahlström, U., & Alehagen, U. (2013). Effect of selenium and Q10 on the cardiac biomarker NT-proBNP. Scandinavian Cardiovascular Journal: SCJ, 47(5), 281-288.
Johansson, P., Dahlström, Ö., Dahlström, U., & Alehagen, U. (2015). Improved health-related quality of life, and more days out of hospital with supplementation with selenium and Coenzyme Q10 combined. Results from a double blind, placebo- controlled prospective study. The Journal of Nutrition, Health & Aging, 19(9), 870-877.
Ju, J., Picinich, S. C., Yang, Z., Zhao, Y., Suh, N., Kong, A., & Yang, C. S. (2010). Cancer-preventive activities of tocopherols and tocotrienols. Carcinogenesis, 31(4), 533-542.
Kaneko, J. J., & Ralston, N. C. (2007). Selenium and mercury in pelagic fish in the central north pacific near Hawaii. Biological Trace Element Research, 119(3), 242-254.
Karamali, M., Nourgostar, S., Zamani, A., Vahedpoor, Z., & Asemi, Z. (2015). The favourable effects of long-term selenium supplementation on regression of cervical tissues and metabolic profiles of patients with cervical intraepithelial neoplasia: a randomised, double-blind, placebo-controlled trial. The British Journal of Nutrition, 114(12), 2039-2045.
Kesse-Guyot, E., Fezeu, L., Jeandel, C., Ferry, M., Andreeva, V., Amieva, H., & Galan, P. (2011). French adults' cognitive performance after daily supplementation with antioxidant vitamins and minerals at nutritional doses: a post hoc analysis of the Supplementation in Vitamins and Mineral Antioxidants (SU.VI.MAX) trial. The American Journal of Clinical Nutrition, 94(3), 892-899.
Klein, E. A., Thompson, I. J., Tangen, C. M., Crowley, J. J., Lucia, M. S., Goodman, P. J., & Baker, L. H. (2011). Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA, 306(14), 1549-1556.
Kohrle J., Jakob F., Contempre B. & Dumont J.E. (2005). Selenium, the thyroid, and the endocrine system. Endocrine Reviews, 2005 26 944–984.
Kristal, A. R., Darke, A. K., Morris, J. S., Tangen, C. M., Goodman, P. J., Thompson, I. M., & ... Klein, E. A. (2014). Baseline selenium status and effects of selenium and vitamin e supplementation on prostate cancer risk. Journal of The National Cancer Institute, 106(3), djt456.
Krysiak, R., & Okopien, B. (2011). The effect of levothyroxine and selenomethionine on lymphocyte and monocyte cytokine release in women with Hashimoto's thyroiditis. The Journal of Clinical Endocrinology and Metabolism, 96(7), 2206-2215.
Kuklinski, B., Pietschmann, A., & Otterstein, A. (1992). Protektion des UV- lichtinduzierten oxidativen Stresses durch nutritive Radikalfänger. Zeitschrift fur die gesamte innere Medizin, 47, 514-518.
Kupka, R., Mugusi, F., Aboud, S., Msamanga, G. I., Finkelstein, J. L., Spiegelman, D., & Fawzi, W. W. (2008). Randomized, double-blind, placebo-controlled trial of selenium supplements among HIV-infected pregnant women in Tanzania: effects on maternal and child outcomes. The American Journal of Clinical Nutrition, 87(6), 1802-1808.
Kupka, R., Mugusi, F., Aboud, S., Hertzmark, E., Spiegelman, D., & Fawzi, W. W. (2009). Effect of selenium supplements on hemoglobin concentration and morbidity among HIV-1-infected Tanzanian women. Clinical Infectious Diseases, 48(10), 1475-1478.
Laclaustra M, Navas-Acien A, Stranges S, Ordovas JM, Guallar E. (2009). Serum selenium concentrations and diabetes in U.S. adults: National Health and Nutrition Examination Survey (NHANES) 2003-2004. Environ Health Perspect, 117(9):1409-1413.
Larmane, L., Zvagule, T., Skesters, A., Silova, A., Rainsford, K., Rusakova, N., & Reste, J. (2008). Effects of long-term antioxidant (selenium and vitamin E) and ibuprofen antioxidant therapy on oxidative stress. Cell Biology and Toxicology, 24(1), S25.
Larsen, E. H., Sloth, J., Hansen, M., & Moesgaard, S. (2003). Selenium speciation and isotope composition in 77-Se enriched yeast using gradient elution HPLC separation and ICP-dynamic reaction cell MS. Journal of Analytical Atomic Spectrometry, 18, 310-316.
Larsen, E. H., Hansen, M., Paulin, H., Moesgaard, S., Reid, M., & Rayman, M. (2004). Speciation and bioavailability of selenium in yeast-based intervention agents used in cancer chemoprevention studies. Journal of AOAC International, 87(1), 225-232.
Lee, E., Myung, S., Jeon, Y., Kim, Y., Chang, Y. J., Ju, W., & ... Huh, B. Y. (2011). Effects of selenium supplements on cancer prevention: meta-analysis of randomized controlled trials. Nutrition and Cancer, 63(8), 1185-1195.
Lei C., Niu X., Wei J., Zhu J., & Zhu Y. (2009). Interaction of glutathione peroxidase-1 and selenium in endemic dilated cardiomyopathy. Clin Chim Acta, 399(1-2):102-108.
Leong, J., van der Merwe, J., Pepe, S., Bailey, M., Perkins, A., Lymbury, R., & Rosenfeldt, F. (2010). Perioperative metabolic therapy improves redox status and outcomes in cardiac surgery patients: a randomised trial. Heart, Lung & Circulation, 19(10), 584-591.
Li, W., Zhu, Y., Yan, X., Zhang, Q., Li, X., Ni, Z., & ... Zhu, J. (2000). [The prevention of primary liver cancer by selenium in high risk populations]. Zhonghua Yu Fang Yi Xue Za Zhi [Chinese Journal of Preventive Medicine], 34(6), 336-338.
Li, Y., Dong, Z., Chen, C., Li, B., Gao, Y., Qu, L., & Chai, Z. (2012). Organic selenium supplementation increases mercury excretion and decreases oxidative damage in long-term mercury-exposed residents from Wanshan, China. Environmental Science & Technology, 46(20), 11313-11318.
Lippman, S. M., Klein, E. A., Goodman, P. J., Lucia, M. S., Thompson, I. M., Ford, L. G., & ... Coltman, C. J. (2009). Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). Jama, 301(1), 39-51.
Lobo, V., Patil, A., Phatak, A., & Chandra, N. (2010). Free radicals, antioxidants and functional foods: Impact on human health. Pharmacognosy Reviews, 4(8), 118-126. Lockhart-Mummery, J. P. (1935). Modern views on the cancer problem. British Medical Journal, 1(3877): 867-869.
Loef, M., Schrauzer, G. N., & Walach, H. (2011). Selenium and Alzheimer's disease: a systematic review. Journal of Alzheimer's Disease, 26(1), 81-104.
Lü, J., Zhang, J., Jiang, C., Deng, Y., Özten, N., & Bosland, M. C. (2016). Cancer chemoprevention research with selenium in the post-SELECT era: Promises and challenges. Nutrition and Cancer, 68(1), 1-17.
MacFarquhar, J. K., Broussard, D. L., Melstrom, P., Hutchinson, R., Wolkin, A., Martin, C., & ... Jones, T. F. (2010). Acute selenium toxicity associated with a dietary supplement. Archives of Internal Medicine, 170(3), 256-261.
Mao, S., Zhang, A., & Huang, S. (2014). Selenium supplementation and the risk of type 2 diabetes mellitus: a meta-analysis of randomized controlled trials. Endocrine, 47(3), 758-763.
Mao, J., Pop, V. J., Bath, S. C., Vader, H. L., Redman, C. G., & Rayman, M. P. (2016). Effect of low-dose selenium on thyroid autoimmunity and thyroid function in UK pregnant women with mild-to-moderate iodine deficiency. European Journal of Nutrition, 55(1), 55-61.
Mao, J., Vanderlelie, J. J., Perkins, A. V., Redman, C. G., Ahmadi, K. R., & Rayman, M. P. (2016). Genetic polymorphisms that affect selenium status and response to selenium supplementation in United Kingdom pregnant women. The American Journal of Clinical Nutrition, 103(1), 100-106.
Mao, J., Bath, S. C., Vanderlelie, J. J., Perkins, A. V., Redman, C. G., & Rayman, M. P. (2016). No effect of modest selenium supplementation on insulin resistance in UK pregnant women, as assessed by plasma adiponectin concentration. The British Journal of Nutrition, 115(1), 32-38.
Marcocci, C., Kahaly, G. J., Krassas, G. E., Bartalena, L., Prummel, M., Stahl, M., & … Wiersinga, W. (2011). Selenium and the Course of Mild Graves' Orbitopathy. New England Journal Of Medicine, 364(20), 1920-1931.
Marshall, J. R., Tangen, C. M., Sakr, W. A., Wood, D. J., Berry, D. L., Klein, E. A., & Thompson, I. J. (2011). Phase III trial of selenium to prevent prostate cancer in men with high-grade prostatic intraepithelial neoplasia: SWOG S9917. Cancer Prevention Research (Philadelphia, Pa.), 4(11), 1761-1769.
McCann JC, Ames BN. (2011). Adaptive dysfunction of selenoproteins from the perspective of the triage theory: why modest selenium deficiency may increase risk of diseases of aging. FASEB J;25(6):1793-814.
Moesgaard, S. & Morrill, R. (2001). The need for speciation to realise the potential of selenium in disease prevention. In L. Ebdon & L. Pitts, (Eds.), Trace Element speciation for Environment, Food, and Health (pp. 261-284). Cambridge, UK: The Royal Society of Chemistry.
Mokhber, N., Namjoo, M., Tara, F., Boskabadi, H., Rayman, M. P., Ghayour- Mobarhan, M., & ... Ferns, G. (2011). Effect of supplementation with selenium on postpartum depression: a randomized double-blind placebo-controlled trial. The Journal of Maternal-Fetal & Neonatal Medicine, 24(1), 104-108.
National Institutes of Health. (2016). Selenium: dietary supplement fact sheet. National Institutes of Health. Office of Dietary Supplements. Retrieved from https://ods.od.nih.gov/factsheets/Selenium-HealthProfessional/ Negro, R., Greco, G., Mangieri, T., Pezzarossa, A., Dazzi, D., & Hassan, H. (2007).
The influence of selenium supplementation on postpartum thyroid status in pregnant women with thyroid peroxidase autoantibodies. The Journal of Clinical Endocrinology and Metabolism, 92(4), 1263-1268.
Nève, J. (2000). New approaches to assess selenium status and requirement. Nutrition Reviews, 58(12), 363-369.
Nylander, M., Friberg, L., & Lind, B. (1987). Mercury concentrations in the human brain and kidneys in relation to exposure from dental amalgam fillings. Swedish Dental Journal, 11(5), 179-187.
Oldfield, J.E. (2002). A brief history of selenium research. Journal of Anim. Science. [Online] Retrieved from https://www.asas.org/docs/publications/oldfieldhist.pdf?sfvrsn=0
Orrell, R. W., Lane, R. M., & Ross, M. (2008). A systematic review of antioxidant treatment for amyotrophic lateral sclerosis/motor neuron disease. Amyotrophic Lateral Sclerosis, 9(4), 195-211.
Outzen, M., Tjønneland, A., Larsen, E. H., Friis, S., Larsen, S. B., Christensen, J., & Olsen, A. (2016). Selenium status and risk of prostate cancer in a Danish population. The British Journal of Nutrition, 115(9), 1669-1677.
Park, K., Rimm, E. B., Siscovick, D. S., Spiegelman, D., Manson, J. E., Morris, J. S., & Mozaffarian, D. (2012). Toenail selenium and incidence of type 2 diabetes in U.S. men and women. Diabetes Care, 35(7), 1544-1551.
Peters, T. L., Beard, J. D., Umbach, D. M., Allen, K., Keller, J., Mariosa, D., & Kamel, F. (2016). Blood levels of trace metals and amyotrophic lateral sclerosis. Neurotoxicology, 54119-126.
Pillai, R., Uyehara-Lock, J. H., & Bellinger, F. P. (2014). Selenium and selenoprotein function in brain disorders. IUBMB Life, 66(4), 229-239.
Pieczyńska, J., & Grajeta, H. (2015). The role of selenium in human conception and pregnancy. Journal of Trace Elements in Medicine and Biology, 2931-38.
Prasad, M. P., Mukundan, M. A., & Krishnaswamy, K. (1995). Micronuclei and carcinogen DNA adducts as intermediate end points in nutrient intervention trial of precancerous lesions in the oral cavity. European Journal of Cancer. Part B, Oral Oncology, 31B(3), 155-159.
Ralston, N.V. & Raymond, L.J. (2010). Dietary selenium's protective effects against methylmercury toxicity. Toxicology, 278(1), 112-23.
Ravn-Haren, G., Krath, B. N., Overvad, K., Cold, S., Moesgaard, S., Larsen, E. H., & Dragsted, L. O. (2008). Effect of long-term selenium yeast intervention on activity and gene expression of antioxidant and xenobiotic metabolising enzymes in healthy elderly volunteers from the Danish Prevention of Cancer by Intervention by Selenium (PRECISE) pilot study. The British Journal of Nutrition, 99(6), 1190-1198.
Rayman, M. P. (2000). The importance of selenium to human health. Lancet, 356(9225), 233-241.
Rayman, M. P., & Rayman, M. P. (2002). The argument for increasing selenium intake. The Proceedings of The Nutrition Society, 61(2), 203-215.
Rayman, M. P. (2005). Selenium in cancer prevention: a review of the evidence and mechanism of action. The Proceedings of The Nutrition Society, 64(4), 527-542.
Rayman M.P., Thompson A., Warren-Perry M., Galassini R., Catterick J., Hall E., & Lawrence D., Bliss J. (2006). Impact of selenium on mood and quality of life: a randomized, controlled trial. Biol. Psychiatry, 59, 2, 147-154.
Rayman M.P., Thompson A.J., Bekaert B., Catterick J., Galassini R., Hall E., Warren-Perry M., Beckett G.J. (2008). Randomized controlled trial of the effect of selenium supplementation on thyroid function in the elderly in the United Kingdom., Am. J. Clin. Nutr., 87, 2, 370-378.
Rayman, M. P. (2009). Selenoproteins and human health: insights from epidemiological data. Biochimica Et Biophysica Acta, 1790(11), 1533-1540.
Rayman, M. P., Combs, G. J., & Waters, D. J. (2009). Selenium and vitamin E supplementation for cancer prevention. JAMA, 301(18), 1876, 1877.
Rayman, M. P., Stranges, S., Griffin, B. A., Pastor-Barriuso, R., & Guallar, E. (2011). Effect of supplementation with high-selenium yeast on plasma lipids: a randomized trial. Annals of Internal Medicine, 154(10), 656-665.
Rayman, M. P., & Stranges, S. (2013). Epidemiology of selenium and type 2 diabetes: can we make sense of it? Free Radical Biology & Medicine, 651557-1564.
Rayman, M. P., Searle, E., Kelly, L., Johnsen, S., Bodman-Smith, K., Bath, S. C., & ...Redman, C. G. (2014). Effect of selenium on markers of risk of pre-eclampsia in UK pregnant women: a randomised, controlled pilot trial. The British Journal of Nutrition, 112(1), 99-111.
Rayman, M. P., Bath, S. C., Westaway, J., Williams, P., Mao, J., Vanderlelie, J. J., & Redman, C. G. (2015). Selenium status in U.K. pregnant women and its relationship with hypertensive conditions of pregnancy. The British Journal of Nutrition, 113(2),249-258.
Rees, K., Hartley, L., Day, C., Flowers, N., Clarke, A., & Stranges, S. (2013). Selenium supplementation for the primary prevention of cardiovascular disease. The Cochrane Database of Systematic Reviews, (1), CD009671.
Reid, M. E., Duffield-Lillico, A. J., Garland, L., Turnbull, B. W., Clark, L. C., & Marshall, J. R. (2002). Selenium supplementation and lung cancer incidence: an update of the nutritional prevention of cancer trial. Cancer Epidemiology, Biomarkers & Prevention, 11(11), 1285-1291.
Reid, M. E., Duffield-Lillico, A. J., Sunga, A., Fakih, M., Alberts, D. S., & Marshall, J. R. (2006). Selenium supplementation and colorectal adenomas: an analysis of the nutritional prevention of cancer trial. International Journal of Cancer, 118(7), 1777-1781.
Reid, M. E., Duffield-Lillico, A. J., Slate, E., Natarajan, N., Turnbull, B., Jacobs, E., & Marshall, J. R. (2008). The nutritional prevention of cancer: 400 mcg per day selenium treatment. Nutrition and Cancer, 60(2), 155-163.
Richie, J. J., Das, A., Calcagnotto, A. M., Sinha, R., Neidig, W., Liao, J., & El- Bayoumy, K. (2014). Comparative effects of two different forms of selenium on oxidative stress biomarkers in healthy men: a randomized clinical trial. Cancer Prevention Research (Philadelphia, Pa.), 7(8), 796-804.
Rotruck, J. T., Pope, A. L., Ganther, H. E., Swanson, A. B., Hafeman, D. G., & Hoekstra, W. G. (1973). Selenium: biochemical role as a component of glutathione peroxidase. Science, 179(4073), 588-590.
Santos, J. R., Gois, A. M., Mendonça, D. F., & Freire, M. M. (2014). Nutritional status, oxidative stress and dementia: the role of selenium in Alzheimer's disease. Frontiers in Aging Neuroscience, 6:206.
Schöpfer, J., Drasch, G., & Schrauzer, G. N. (2010). Selenium and cadmium levels and ratios in prostates, livers, and kidneys of nonsmokers and smokers. Biological Trace Element Research, 134(2), 180-187.
Schrauzer, G. N. et al. (1977). Cancer mortality correlation studies III: statistical associations with dietary selenium intakes. Bioinorganic Chemistry, 7(1), 23-31.
Schrauzer, G. N., White, D. A., & Schneider, C. J. (1978). Selenium and cancer: effects of selenium and of the diet on the genesis of spontaneous mammary tumors in virgin inbred female C3H/St mice. Bioinorganic Chemistry, 8(5), 387-396.
Schrauzer, G. N. (2000). Anticarcinogenic effects of selenium. Cellular and Molecular Life Sciences: CMLS, 57(13-14), 1864-1873.
Schrauzer, G. N. (2001). Nutritional selenium supplements: product types, quality, and safety. Journal of The American College Of Nutrition, 20(1), 1-4.
Schrauzer G (2006). "Selenium yeast: composition, quality, analysis, and safety". Pure Appl Chem 78: 105–109.
Schrauzer, G. N. (2009). RE: Lessons from the selenium and vitamin E cancer prevention trial (SELECT). Critical Reviews in Biotechnology, 29(2), 81.
Schrauzer, G. N. (2009). Selenium and selenium-antagonistic elements in nutritional cancer prevention. Critical Reviews in Biotechnology, 29(1), 10-17.
Schrauzer, G. N., & Surai, P. F. (2009). Selenium in human and animal nutrition: resolved and unresolved issues. A partly historical treatise in commemoration of the fiftieth anniversary of the discovery of the biological essentiality of selenium, dedicated to the memory of Klaus Schwarz (1914-1978) on the occasion of the thirtieth anniversary of his death. Critical Reviews in Biotechnology, 29(1), 2-9.
Schwarz, K. & Foltz, C. M. (1957). Selenium as an integral part of factor 3 against dietary necrotic liver degeneration." Journal of the American Chemical Society 79 (12): 3292–3293.
Seppänen, K., Kantola, M., Laatikainen, R., Nyyssönen, K., Valkonen, V. P., Kaarlöpp, V., & Salonen, J. T. (2000). Effect of supplementation with organic selenium on mercury status as measured by mercury in pubic hair. Journal of Trace Elements in Medicine and Biology (GMS), 14(2), 84-87.
Shahar, A., Patel, K. V., Semba, R. D., Bandinelli, S., Shahar, D. R., Ferrucci, L., & Guralnik, J. M. (2010). Plasma selenium is positively related to performance in neurological tasks assessing coordination and motor speed. Movement Disorders, 25(12), 1909-1915.
Shamberger, R. J., & Frost, D. V. (1969). Possible protective effect of selenium against human cancer. Canadian Medical Association Journal, 100(14), 682.
Skesters, A., Zvagule, T., Larmane, L., Rainsford, K., Silova, A., Rusakova, N., Mustafins, P. (2008). Effects of selenium alone and with antioxidants and ibuprofen mixture in Chernobyl catastrophe clean-up workers at risk of developing cancer. Cell Biology and Toxicology, 24(1), S31.
Sperduto, R. D., Hu, T. S., Milton, R. C., Zhao, J. L., Everett, D. F., & Cheng, Q. F., Blot, W.J.; Bing, L., Taylor, P.R., & Li, J.Y. (1993). The Linxian cataract studies. Two nutrition intervention trials. Archives of Ophthalmology, 111(9), 1246-1253.
Steinbrenner, H. (2013). Interference of selenium and selenoproteins with the insulin-regulated carbohydrate and lipid metabolism. Free Radical Biology & Medicine, 651538-1547.
Stone, C. A., Kawai, K., Kupka, R., and Fawzi, W. W. (2010). Role of selenium in HIV infection. Nutrition Reviews 68 (11) (October 20): 671–681.
Stranges, S., Marshall, J. R., Trevisan, M., Natarajan, R., Donahue, R. P., Combs, G.F., & Reid, M. E. (2006). Effects of selenium supplementation on cardiovascular disease incidence and mortality: secondary analyses in a randomized clinical trial. American Journal of Epidemiology, 163(8):694-699.
Stranges, S., Marshall JR, Natarajan R, et al. (2007). Effects of long-term selenium supplementation on the incidence of type 2 diabetes: a randomized trial. Ann Intern Med, 147(4):217-223.
Stranges, S., Sieri, S., Vinceti, M., Grioni, S., Guallar, E., Laclaustra, M., & Krogh, V. (2010). A prospective study of dietary selenium intake and risk of type 2 diabetes. BMC Public Health, 10:564.
Tara, F., Rayman, M. P., Boskabadi, H., Ghayour-Mobarhan, M., Sahebkar, A., Alamdari, D. H., & Ferns, G. (2010). Prooxidant-antioxidant balance in pregnancy: a randomized double-blind placebo-controlled trial of selenium supplementation. Journal of Perinatal Medicine, 38(5), 473-478.
Tara, F., Maamouri, G., Rayman, M. P., Ghayour-Mobarhan, M., Sahebkar, A., Yazarlu, O., & Ferns, G. (2010). Selenium supplementation and the incidence of preeclampsia in pregnant Iranian women: a randomized, double-blind, placebo- controlled pilot trial. Taiwanese Journal of Obstetrics & Gynecology, 49(2), 181-187.
Tara, F., Rayman, M. P., Boskabadi, H., Ghayour-Mobarhan, M., Sahebkar, A., Yazarlu, O., & Ferns, G. (2010). Selenium supplementation and premature (pre- labour) rupture of membranes: a randomised double-blind placebo-controlled trial. Journal of Obstetrics and Gynaecology, 30(1), 30-34.
Thompson, P., Roe, D. J., Fales, L., Buckmeier, J., Wang, F., Hamilton, S. R., & Lance, P. (2012). Design and baseline characteristics of participants in a phase III randomized trial of celecoxib and selenium for colorectal adenoma prevention. Cancer Prevention Research (Philadelphia, Pa.), 5(12), 1381-1393.
Thomson, C. D. (1998). Selenium speciation in human body fluids. The Analyst, 123(5), 827-831.
Tolonen, M., Sarna, S., Halme, M., Tuominen, S. E., Westermarck, T., Nordberg, U. R., & Schrijver, J. (1988). Anti-oxidant supplementation decreases TBA reactants in serum of elderly. Biological Trace Element Research, 17221-228.
Tolonen, M. (1990). Vitamins and minerals in health and nutrition. New York: Ellis Horwood.
Tsuji, P. A. (2015). Selenium. Linus Pauling Institute. Micronutrient Information Center. Retrieved from http://lpi.oregonstate.edu/mic/minerals/selenium.
Turker, O., Kumanlioglu, K., Karapolat, I., & Dogan, I. (2006). Selenium treatment in autoimmune thyroiditis: 9-month follow-up with variable doses. The Journal of Endocrinology, 190(1), 151-156.
Uttara, B., Singh, A. V., Zamboni, P., & Mahajan, R. T. (2009). Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Current Neuropharmacology, 7(1), 65-74.
Van Zuuren, E. J., Albusta, A. Y., Fedorowicz, Z., Carter, B., & Pijl, H. (2013). Selenium supplementation for Hashimoto's thyroiditis. The Cochrane Database of Systematic Reviews, (6), CD010223.
Venardos, K., Harrison, G., Headrick, J., & Perkins, A. (2004). Effects of dietary selenium on glutathione peroxidase and thioredoxin reductase activity and recovery from cardiac ischemia-reperfusion. Journal of Trace Elements In Medicine And Biology, 18(1), 81-88.
Vinceti, M., Crespi, C. M., Malagoli, C., Del Giovane, C., & Krogh, V. (2013). Friend or foe? The current epidemiologic evidence on selenium and human cancer risk. Journal of Environmental Science and Health. Part C, Environmental Carcinogenesis & Ecotoxicology Reviews, 31(4), 305-341.
Voicehovska, J., Skesters, A., Orlikov, G. A., Silova, A. A., Rusakova, N. E., Larmane, L. T., & Maulinsh, E. (2008). Assessment of some oxidative stress parameters in bronchial asthma patients after selenium supplementation. Biochemistry (Moscow) Supplement Series B: Biomedical Chemistry, 2,2, 189–193.
Wang, X., Yang, T., Wei, J., Lei, G., & Zeng, C. (2016). Association between serum selenium level and type 2 diabetes mellitus: a non-linear dose-response meta- analysis of observational studies. Nutrition Journal, 15(1), 48.
Watt, T., Cramon, P., Bjorner, J. B., Bonnema, S. J., Feldt-Rasmussen, U., Gluud, C., & Rasmussen, A. K. (2013). Selenium supplementation for patients with Graves' hyperthyroidism (the GRASS trial): study protocol for a randomized controlled trial. Trials, 14:119.
Weekley, C. M., & Harris, H. H. (2013). Which form is that? The importance of selenium speciation and metabolism in the prevention and treatment of disease. Chemical Society Reviews, 42(23), 8870-8894.
Wei, H. J. (1989). [Influence of selenium supplement on cadmium metabolism in human]. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. Acta Academiae Medicinae Sinicae, 11(3),185-189.
Wei, W., Abnet, C. C., Qiao, Y., Dawsey, S. M., Dong, Z., Sun, X., & ... Mark, S. D. (2004). Prospective study of serum selenium concentrations and esophageal and gastric cardia cancer, heart disease, stroke, and total death. The American Journal of Clinical Nutrition, 79(1), 80-85.
Wei, J., Zeng, C., Gong, Q., Yang, H., Li, X., Lei, G., & Yang, T. (2015). The association between dietary selenium intake and diabetes: a cross-sectional study among middle-aged and older adults. Nutrition Journal, 1418.
Weiner, J. A., Nylander, M., & Berglund, F. (1990). Does mercury from amalgam restorations constitute a health hazard?. The Science of the Total Environment, 99(1-2), 1-22.
Weiner, J. A., & Nylander, M. (1995). An estimation of the uptake of mercury from amalgam fillings based on urinary excretion of mercury in Swedish subjects. The Science of the Total Environment, 168(3), 255-265.
Westermarck, T., Tolonen, M., Sarna, S., Halme, M., Tuominen, S. E., Keinonen, M., & Nordberg, U. R. (1988). Antioxidant supplementation for elderly living in a nursing home - a double blind randomized one year clinical trial. Trace Elements in Man and Animals, vol 6. New York: Plenum Publishing.
Westermarck, T, Sauka, M., Selga, G., Skesters, A., & Abdulla, M., & Atroshi, F. (2008). Effects of cocktail antioxidant supplementation on oxidative stress in AIDS. Cell Biology and Toxicology, 24(1), S55-S56.
Whanger, P. D., Xia, Y., & Thomson, C. D. (1994). Protein technics for selenium speciation in human body fluids. Journal of Trace Elements and Electrolytes in Health And Disease, 8(1), 1-7.
Whanger, P. D. (2002). Selenocompounds in plants and animals and their biological significance. Journal of The American College of Nutrition, 21(3), 223-232.
What you need to know about mercury in fish and shellfish. (2014). U.S. Food and Drug Administration. Retrieved from http://www.fda.gov/Food/ResourcesForYou/Consumers/ucm110591.htm
Winther KH, Watt T, Bjorner JB, et al. (2014). The chronic autoimmune thyroiditis quality of life selenium trial (CATALYST): study protocol for a randomized controlled trial. Trials, 15:115.
Winther, K. H., Bonnema, S. J., Cold, F., Debrabant, B., Nybo, M., Cold, S., & Hegedüs, L. (2015). Does selenium supplementation affect thyroid function? Results from a randomized, controlled, double-blinded trial in a Danish population. European Journal of Endocrinology / European Federation of Endocrine Societies, 172(6), 657-667.
Witte, K. A., Nikitin, N. P., Parker, A. C., von Haehling, S., Volk, H., Anker, S. D., & Cleland, J. F. (2005). The effect of micronutrient supplementation on quality-of- life and left ventricular function in elderly patients with chronic heart failure. European Heart Journal, 26(21), 2238-2244.
Xu, M., Guo, D., Gu, H., Zhang, L., & Lv, S. (2016). Selenium and Preeclampsia: a Systematic Review and Meta-analysis. Biological Trace Element Research, 171(2), 283-292.
Yadav, V., Shinto, L., & Bourdette, D. (2010). Complementary and alternative medicine for the treatment of multiple sclerosis. Expert Review of Clinical Immunology, 6(3), 381-395.
Yang, G. Q., Ge, K. Y., Chen, J. S., & Chen, X. S. (1988). Selenium-related endemic diseases and the daily selenium requirement of humans. World Review of Nutrition and Dietetics, 5598-152.
Yu, S. Y., Zhu, Y. J., & Li, W. G. (1997). Protective role of selenium against hepatitis B virus and primary liver cancer in Qidong. Biological Trace Element Research, 56(1), 117-124.
Zhang, X., Liu, C., Guo, J., & Song, Y. (2016). Selenium status and cardiovascular diseases: meta-analysis of prospective observational studies and randomized controlled trials. European Journal of Clinical Nutrition, 70(2), 162-169.
Zou K., Liu G., Wu T., & Du L. (2009). Selenium for preventing Kashin-Beck osteoarthropathy in children: a meta-analysis. Osteoarthritis Cartilage, 17(2):144- 151.

