Why choose a Clinically Documented CoQ10?
The absorption and the efficacy of CoQ10 supplements vary considerably depending on the formulation. CoQ10 supplements produced from the best CoQ10 raw material will give a poor absorption if the manufacturer has not used a proven formulation process.
Whether you are a consumer, a researcher or a medical doctor, you will want to use a CoQ10 supplement with documented absorption and proven effects in clinical studies.
 A randomized controlled study completed in 2018 has shown that Pharma Nord's Bio-Quinone Active CoQ10 has double the absorption of the second best tested formulation and three to ten times better absorption than the other five tested formulations [Lopez-Lluch].
A randomized controlled study completed in 2018 has shown that Pharma Nord's Bio-Quinone Active CoQ10 has double the absorption of the second best tested formulation and three to ten times better absorption than the other five tested formulations [Lopez-Lluch].
All products contained the same amount of CoQ10, but there was a statistically significant difference as to how much CoQ10 was absorbed from each product.
"This is important because the CoQ10 cannot protect your heart muscle cells if it is not absorbed into the blood circulation. "
The KiSel-10 and Q-Symbio studies document a protective effect of supplementation with Bio-Quinone Active CoQ10 on heart function in senior citizens and in heart failure patients respectively [Alehagen; Mortensen].
Follow in the Path of Leading CoQ10 Researchers
Researchers are becoming increasingly aware of the importance of using reliable and bioavailable products for clinical trials. Two large international CoQ10 clinical trials have stood out, not just because the researchers chose to use Pharma Nord's acclaimed CoQ10 product for their research, but because the clinical trials actually showed a statistically significant effect from CoQ10 supplementation in both cases.
These clinical trials have helped emphasize the importance of selecting a CoQ10 product with documented bioavailability.
One of the clinical trials has been named KiSel-10 and was done by Swedish cardiologists from the University of Linköping and from the Karolinska Institute in Stockholm. The clinical trial results were published in 2014 in the International Journal of Cardiology.
The second clinical trial, Q-Symbio, was headed by the late cardiology researcher, Dr. Svend Aage Mortensen, from Rigshospitalet (state hospital) in Copenhagen and the results were published a year later in the Journal of the American College of Cardiology, Heart Failure, which is one of the world’s leading medical journals.
Clinical trials like these show that the Pharma Nord CoQ10 used is bioavailable and absorbed by participants. If it were not the case, there would not be a measurable effect from the CoQ10 supplementation as compared to the placebo group.
A Primary and a Secondary Endpoint from the Q-Symbio Study
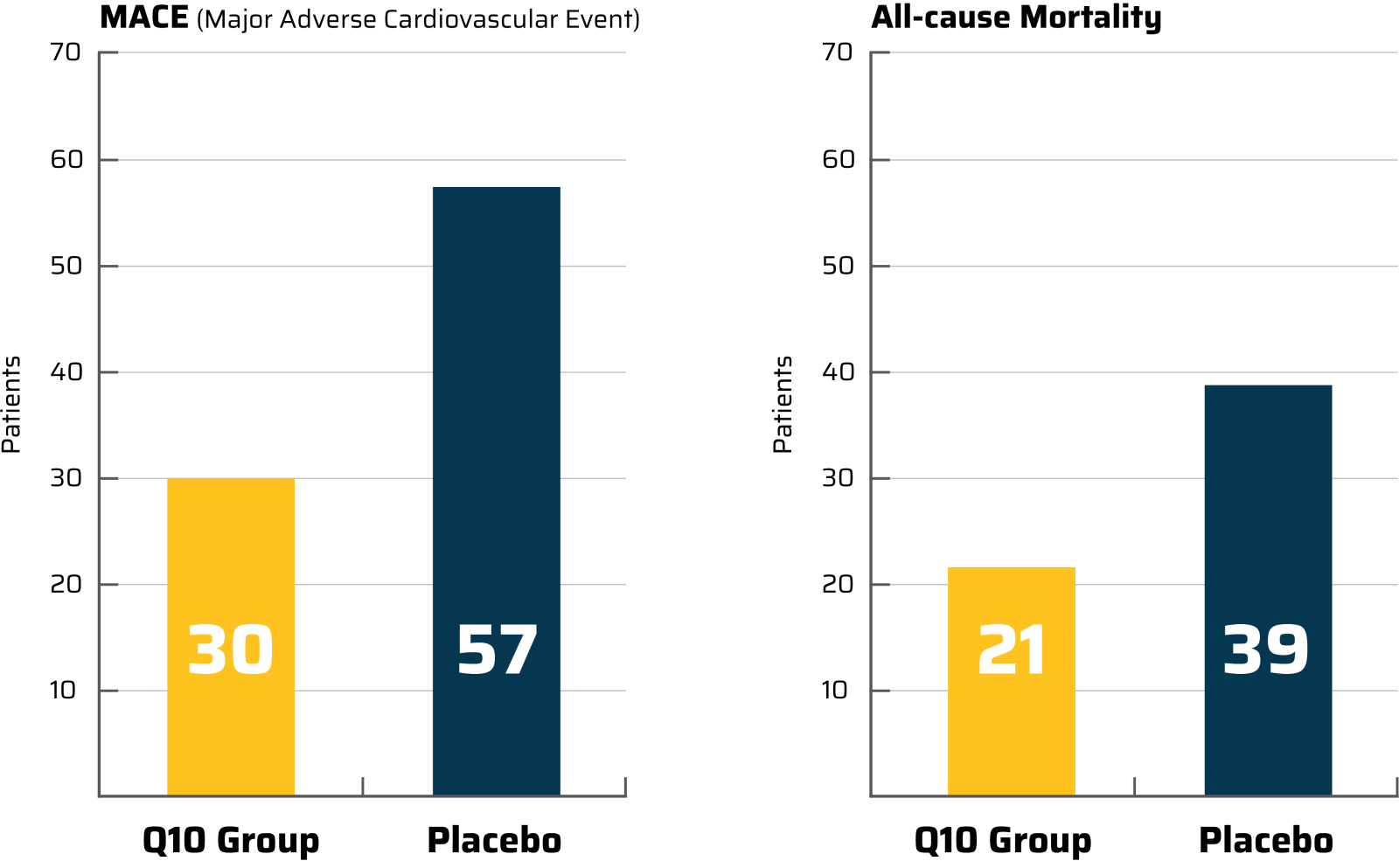
MACE was a primary endpoint of the Q-Symbio study. This was an endpoint for which the study participants were randomized and for which the trial was powered.
All-cause Mortality was a secondary endpoint because this statistical analyse was not specified before the data was seen.
Ask your CoQ10 Manufacturer which Clinical Trials they have Supplied
Your CoQ10 manufacturer should be able to readily provide evidence about the clinical studies that were conducted using their CoQ10 formulation. These studies should show a significant and positive effect from the CoQ10 supplementation. Otherwise, it could be a sign that the manufacturer has not supplied any clinical studies or that the formulation provided had poor effect because it was not absorbed.
Ever since Pharma Nord launched its first CoQ10 product in 1991 (Bio-Qinon Q10 - in the US this product is named Bio-Quinone Active CoQ10 Gold), the manufacturer has made it a point to inform its consumers and the industry about the importance of using clinically documented products.
Pharma Nord always strives to encourage the individual consumer to ask us and other manufacturers for documentation. Bio-Quinone Active CoQ10 Gold has been involved in 78 human clinical trials, and we want you to know how it has performed in these independent and published clinical trials. Our message about quality and documentation has had a big influence on both consumers and researchers alike.
"At the end of the day, scientific research using a product the body cannot absorb has already failed before the product is ingested. "
In connection with products with absorption issues, we find it relevant to ask whether the clinical trials that were unable find any positive effects from CoQ10 supplementation simply were using poor products that were unabsorbable?
How to evaluate CoQ10 studies
What documentation will you expect from the CoQ10 supplement manufacturer? At the very least, you will want information about the following aspects of the scientific studies:
Publication: Where was the study published?
Methodology: Was the study a randomized, double-blind, placebo-controlled study? Such studies are the gold standard of experimental research.
Sample size: How many people participated in the study? How homogeneous were the study participants? It is difficult to generalize from the results of wildly heterogeneous studies.
Dosage: How many milligrams of CoQ10 did the study participants take each day? Were the CoQ10 doses taken with a meal? Were the CoQ10 doses divided throughout the day?
Duration: How long did the period of supplementation last?
Results: Did the blood CoQ10 concentrations in the active CoQ10 treatment group improve significantly better than in the placebo control group or in the comparison group?
Please remember: It all comes down to one thing and one thing only: Absorption. If the active ingredient never makes it into your bloodstream and from there into your cells, there is no way the product can have a positive effect.
Coenzyme Q10 supplements are far from equal in their absorbability and their efficacy. Consequently, it is best to choose a Coenzyme Q10 supplement with absorption and effects documented in clinical trials.
Bio-QuinoneTM Active CoQ10 is the nutritional supplement version of Myoqinon, the Coenzyme Q10 preparation licensed for adjuvant treatment of chronic heart failure in a European Union member country. Both variants are ubiquinone Coenzyme Q10 preparations manufactured using a medicine-quality formulation with pharmaceutical-grade raw material, good manufacturing practice, and pre-heating and dissolving of the Coenzyme Q10 crystals in a mixture of vegetable oils prior to encasing in soft-gel capsules.
To date, 78 unique human studies of the absorption, efficacy, and safety of these two preparations have been published in journal articles and conference papers.
Of these 78 studies, 26 are gold standard studies that are randomized, double-blind, placebo-controlled studies enrolling 30 or more study participants.
Some of these studies done with Myoqinon and Bio-QuinoneTM Active CoQ10 are recognized as the most important clinical studies in the Coenzyme Q10 research field: the Q-Symbio Study of the effect of Coenzyme Q10 on morbidity and mortality in chronic heart failure [Mortensen], the KiSel-10 Study of the effect of combined selenium and Coenzyme Q10 supplementation on cardiovascular mortality in senior citizens [Alehagen], and the Gulf War Illness Study of the effect of Coenzyme Q10 on physical function and symptoms in veterans with Gulf War Illness [Golomb] as well as the recent comparative bio-availability study of various Coenzyme Q10 formulations including the Myoqinon formulation [Lopez-Lluch].
 The original CoQ10 product that was used for most of the scientific studies documenting the effects of Q10 was a medical preparation called Myoqinon.
The original CoQ10 product that was used for most of the scientific studies documenting the effects of Q10 was a medical preparation called Myoqinon.
This is now available in the US as a dietary suplement under the name of Bio-Quinone Active. It is manufactured in exactly the same way as Myoqinon and is identical.
Important Bio-Quinone Studies
-
Antioxidant protection
-
Absorption/Bioavailability
-
Cardiovascular mortality
-
Cardiovascular health
-
Diabetes Type I
-
Diabetes Type II
-
Down Syndrome
-
Exercise
-
Fertility
-
Fibromyalgia
-
Friedreich’s ataxis
-
Gold Standard Studies
Mortensen 2014, Golomb 2014, Brauner 2014, Alehagen 2013, Bogsrud 2013, Fedacko 2013, Thakur 2013, Tiano 2011, Balercia 2009, Cooper 2008, Skesters 2008, Lukmann 2007, Singh 2005, Hoenjet 2005, Rabing Christensen 2004 Zita 2003, Lankin 2003, Khatta 2000, Kaikkonen 2000, Zorn 2000, Henriksen 1999, Kaikkonnen 1997, Nylander 1996, Salonen 1996, Alford 1996, Kuklinski 1994 -
Gulf War Illness
-
Heart failure
-
Periodontal disease
-
Sickle cell disease
-
Sperm motility
-
Statin-induced myopathy
-
Unique human studies
López-Lluch 2019, Alcocer-Gómez 2017, Brauner 2014, Golomb 2014, Mortensen 2014, Cordero 2014, Alehagen 2013, Bogsrud 2013, Fedacko 2013, Thakur 2013, Tiano 2011, Spurney 2011, Balercia 2009, Hertz 2009, Cooper 2008, Montini 2008, Nielsen 2008, Skesters 2008, Skough 2008, Tauler 2008, Westermarck 2008, Zmitek 2008, Lukmann 2007, Sindberg 2007, Singh 2005, Soongswang 2005, Hart 2005, Hoenjet 2005, Balercia 2004, Rabing Christensen 2004, Zita 2003, Elshershari 2003, Horstink 2003, Lankin 2003, Nielsen 2003, Engelsen 2002, Lister 2002, Hodges 2002, Soongswang 2002, Turunen 2002, Lodi 2001, sunesen 2001, khatta 2000, kaikkonen 2000, zorn 2000, eriksson 1999, henriksen 1999, munkholm 1999, hodges 1999, denny 1999, watson 1999, mari 1998, tholle1998, aejmelaeus 1997, lewin 1997, ylikoski 1997, serebruany 1997, kaikkonen 1997, mizuno 1997, strijks 1997, weber 1997, alford 1996, alho 1996, Lönnrot 1996, mizuno 1996, nylander 1996, salonen 1996, taggart 1996, alleva 1995, laaksonen 1995, folkers 1994, lockwood 1994, kuklinski 1994, weis 1994, weber 1994, mortensen 1993, nylander 1991 -
Warfarin dosage
| Researcher | Guillermo Lopez-Lluch |
| RESEARCHER AFFILIATION | Pablo de Olavide University in Sevilla, Spain, |
| PUBLICATION CITATION | López-Lluch, G., Del Pozo-Cruz, J., Sánchez-Cuesta, A., Cortés-Rodríguez, A. B., & Navas, P. (2019). Bioavailability of Coenzyme Q10 supplements depends on carrier lipids and solubilization. Nutrition, 57, 133–140. |
| TYPE OF STUDY | Double-blind, cross-over comparative bio-availability study |
| SAMPLE SIZE | 14 young, healthy Spanish men (10) and women (4) citizens aged 18 to 33 |
| DOSAGE | Bio-Quinone Q10 100 mg single dosage compared with 100 mg single dosage of five other ubiquinone CoQ10 formulations and one ubiquinol formulation |
| RESULTS | |
|
The two best absorbed CoQ10 formulations the soft-gel capsules containing Bio-Quinone Q10 ubiquinone (the oxidized form of CoQ10) formulation and the ubiquinol (the reduced form of CoQ10) formulation. The Bio-Quinone Q10 formulation was absorbed significantly better than the ubiquinol formulation and the other five ubiquinone formulations. |
| Researcher | Anne Louise Mortensen |
| RESEARCHER AFFILIATION | Bio-chemist |
| PUBLICATION CITATION | Mortensen, A. L., Rosenfeldt, F. & Filipiak, K. J. (2019). Effect of Coenzyme Q10 in the Europeans with chronic heart failure: a sub-analysis of the Q-Symbio randomized double-blind trial. Cardiology Journal, published online 20 Feb 2019, doi: 10.5603/CJ.a2019.0022, retrieved from https://journals/viamedica.pl/cardiology_journal/article/view/CJ.a2019.0022 |
| TYPE OF STUDY | Two-year multi-center, randomized, double-blind, placebo-controlled clinical trial (sub-analysis of the original 2014 Q-Symbio study) |
| SAMPLE SIZE | 231 European patients out of the 420 heart failure patients |
| DOSAGE | 3 times Bio-Quinone 100 mg per day, with meals (Bio-Quinone called Myoqinon) |
| RESULTS | |
|
Bio-chemist Dr. Anne Louise Mortensen separated out the data for the 231 European heart failure patients who participated in the Q-Symbio study. She compared the data from the more homogeneous European sub-population of the Q-Symbio study with the data from the total Q-Symbio study population, which included Asian and Australian study participants as well. |
| Researcher | Svend Aage Mortensen |
| RESEARCHER AFFILIATION | Copenhagen University Hospital, Denmark |
| PUBLICATION CITATION | Mortensen, S. A., Rosenfeldt, F., Kumar, A., Dolliner, P., Filipiak, K. J., Pella, D., & Littarru, G. P. (2014). The effect of coenzyme Q10 on morbidity and mortality in chronic heart failure: results from Q-SYMBIO: a randomized double-blind trial. JACC. Heart Failure, 2(6), 641-649. |
| TYPE OF STUDY | Two-year multi-center, randomized, double-blind, placebo-controlled clinical trial |
| SAMPLE SIZE | 420 heart failure patients |
| DOSAGE | 3 times Bio-Quinone 100 mg per day, with meals (Bio-Quinone called Myoquinon) |
| RESULTS | |
|
Adjuvant treatment with Myoqinon (Bio-Quinone) resulted in these statistically significant outcomes as compared with placebo treatment. • A 43% decrease in cardiovascular deaths There were fewer side effects in the Q10 group. |
| Researcher | Urban Alehagen |
| RESEARCHER AFFILIATION | Department of Medicine and Health Sciences, Linköping University, Sweden. |
| PUBLICATION CITATION | Alehagen, U., Johansson, P., Björnstedt, M., Rosén, A., & Dahlström, U. (2013). Cardiovascular mortality and N-terminal-proBNP reduced after combined selenium and coenzyme Q10 supplementation: a 5-year prospective randomized double-blind placebo-controlled trial among elderly Swedish citizens. International Journal of Cardiology, 167(5), 1860-1866. |
| TYPE OF STUDY | Four-year randomized, double-blind, placebo-controlled clinical trial |
| SAMPLE SIZE | 443 healthy elderly Swedish citizens aged 70 to 88 |
| DOSAGE | Bio-Quinone Q10 100 mg twice a day Seleno-Precise 200 micrograms once a day |
| RESULTS | |
|
Supplementation with Coenzyme Q10 and selenium resulted in these statistically significant outcomes: • A reduction in cardiovascular deaths To date, there have been additional 13 publications detailing the results of secondary analyses of the data generated by Professor Alehagen's KiSel-10 clinical trial. These 12 publications are summarized on the pages 7 – 19.
In patients with chronic heart failure, the heart secretes a substance that contains two proteins: B-type natriuretic peptide (called BNP) and N-terminal-pro-BNP (called NT-pro-BNP). Rising levels of these two proteins in the blood are indicative of elevated cardiac wall tension and, therefore, of worsening heart failure. Consequently, a relatively simple and inexpensive blood test can be used to detect the levels of these normally reliable bio-markers of chronic heart failure. Johansson, P., Dahlström, Ö., Dahlström, U., & Alehagen, U. (2015). Improved health-related quality of life, and more days out of hospital with supplementation with selenium and coenzyme q10 combined. Results from a double blind, placebo-controlled prospective study. The Journal of Nutrition, Health & Aging, 19(9), 870-877. Continuing to mine the data from the KiSel-10 study, the Swedish researchers investigated the effect of 48 months of supplementation with Q10 and selenium on non-institutionalized elderly citizens with respect to the number of days out of hospital and health-related quality of life (Hr-QoL). They examined the data from all hospital admissions during the study, and they examined the data from a Quality of Life Short Form-36 (SF-36), from the Cardiac Health Profile (CHP), and from a one-item overall-quality-of-life survey.
Having established that a four-year-long daily supplementation with a combination of 200 mg of ubiquinone Q10 and 200 mcg of organic selenium yeast significantly reduces the risk of cardiovascular death in elderly Swedish citizens (average age: 78 years), Professor Alehagen and his team of researchers undertook a secondary analysis of the data from the KiSel-10 clinical trial. The KiSel-10 researchers knew that selenium and Coenzyme Q10 are involved in the body’s antioxidative defense mechanisms. Therefore, they evaluated the effect of selenium and Coenzyme Q10 supplementation on copeptin and adrenomedullin levels in the blood as biomarkers of oxidative stress.
Having established that four years of supplementation with SelenoPrecise and Bio-Quinone Q10 was positively associated with reduced cardiovascular disease risk and with improved cardiac function, the KiSel-10 researchers followed up by analyzing the persistence of the effect of supplementation on cardiovascular mortality 10 years after the initiation of the active treatment.
In this article, Professor Alehagen and Dr. Aaseth undertake a brief review of what they have learned, as clinicians, from the results of the KiSel-10 study and from their review of the literature of Coenzyme Q10 and selenium. The Linköping University researchers knew that the daily dietary intake of selenium is generally quite low in Sweden. They measured and evaluated the serum selenium levels of 668 elderly citizens of the rural municipality of Kinda. They established that the serum selenium concentrations of the study participants in this sample was, on average, 67.1 micrograms per liter. This is a level considerably below the physiological saturation level required for the activation of important selenoproteins such selenoprotein P and the glutathione peroxidases. To show how low the serum selenium concentrations of the elderly Swedish population was, we can compare with the plasma selenium concentrations of 119 healthy British men and women aged 50 – 64 years [Hurst 2010]. The British study participants had a mean plasma selenium level of 95.7 micrograms per liter with a standard deviation of 11.5 micrograms per liter. Roughly 68 percent of the study participants had plasma selenium concentrations that ranged between 84.2 and 107.2 micrograms per liter. Roughly 95 percent of the study participants had plasma selenium concentrations that ranged between 72.7 and 118.7 micrograms per liter. That is to say, higher than the Swedish average to 67.1 micrograms of selenium per liter of serum. The researchers made appropriate adjustments to rule out the effect of such confounding variables as male gender, smoking, diabetes, chronic obstructive pulmonary disease, and impaired heart function. They found that individuals in the lowest quartile of serum selenium concentrations were at a 43% greater risk of death from all causes and at a 56% greater risk of death from heart disease. The researchers concluded that moderate daily supplementation with selenium might improve the overall health of the Swedish population. The KiSel-10 researchers did a secondary analysis of the available study data to determine whether the positive effects of daily supplementation with Coenzyme Q10 and high-selenium yeast for four years are directly associated with the baseline serum selenium levels of study participants. They reported two important findings from their secondary analysis: 2) The daily supplementation with a combination of Coenzyme Q10 and high-selenium yeast was shown to provide protection against death from heart disease in the study participants with baseline serum selenium levels below 85 micrograms per liter. Alehagen, U., Johansson, P., Aaseth, J., Alexander, J., & Wågsäter, D. (2017). Significant changes in circulating microRNA by dietary supplementation of selenium and Coenzyme Q10 in healthy elderly males. A subgroup analysis of a prospective randomized double-blind placebo-controlled trial among elderly Swedish citizens. Plos One, 12(4), e0174880. doi:10.1371/journal.pone.0174880 The KiSel-10 researchers focused in on the data from 50 study participants, all males, 25 of whom had been in the active treatment group and 25 of whom had been in the placebo group. They isolated RNA from the plasma of these 50 study participants. Then they analyzed the pre-treatment and post-treatment levels of the expression of numerous microRNAs. At baseline, there were no significant differences between the two groups in terms of microRNA expression levels. After four years of treatment with either Coenzyme Q10 and high-selenium yeast or placebo, however, the data showed significant differences between the two groups in as many as 70 different microRNAs. (MicroRNAs are non-coding RNA molecules involved in the regulation of genes that code for proteins. These microRNAs can inhibit the expression of genes and thus influence the formation of proteins. MicroRNAs can play a significant role in the development of heart disease and diabetes.) The researchers concluded that the significant differences between the Coenzyme Q10/high-selenium yeast treatment group and the placebo group in the expression of microRNAs might be one of the biological mechanisms by which the supplementation with Coenzyme Q10 and high-selenium yeast reduced significantly the risk of death from heart disease and reduced the extent of inflammation in the elderly Swedish citizens.
The KiSel-10 study data show conclusively that daily Coenzyme Q10 and high-selenium yeast supplements provide protection against death from heart disease and from age-related declines in heart function. The researchers trace these beneficial effects in part to the antioxidant and anti-inflammatory functions of the two substances. Insulin-like growth factor-1 (abbreviated IGF-1) has many functions in the body such as cell growth and metabolism as well as anti-inflammatory and antioxidative effects. IGF-1 concentrations tend to decrease with increasing age and during periods of inflammation. Professor Alehagen and the KiSel-10 researchers examined the effects of four years of daily supplementation with Coenzyme Q10 and high-selenium yeast on concentrations of IGF-1 and its binding protein IGFBP-1 in the elderly Swedish study participants. They found that the individuals in the group taking Coenzyme Q10 and high-selenium yeast supplements had significantly increased IGF-1 and IGF-1 SD scores at the end of the study period while individuals in the placebo group had reduced concentrations. The researchers suggested that the positive effect of Coenzyme Q10 and high-selenium yeast supplementation on IGF-1 concentrations might be one of the biological mechanisms explaining the positive clinical effects the risk of death from heart disease and on heart function. Alehagen, U., Aaseth, J., Alexander, J., Svensson, E., Johansson, P., & Larsson, A. (2017). Less fibrosis in elderly subjects supplemented with selenium and coenzyme Q10-A mechanism behind reduced cardiovascular mortality? Biofactors (Oxford, England), doi:10.1002/biof.1404 Professor Alehagen and his research colleagues investigated the effect of supplementation with Coenzyme Q10 and high-selenium yeast on eight bio-markers of fibrogenic activity in healthy elderly Swedish citizens, aged 70 – 88 years. Note: Cardiac fibrosis is the development of excess fibrous tissue depositions in the heart muscle or heart valves. Cardiac fibrosis can increase the risk of heart failure. The researchers analyzed the blood concentrations of the various bio-markers at the six-month mark and the 42-month mark in the study. The data showed that there were significantly reduced blood concentrations of seven of the eight fibrosis bio-markers in the active Coenzyme Q10 and selenium group after 42 weeks of supplementation as compared with the placebo group. The reduced fibrogenic activity seems to be a consequence of the daily intervention with Coenzyme Q10 and high-selenium supplements. There appears to be an association between the supplementation and the reduction in the bio-markers of fibrosis and the statistically significant reduction in the risk of death from cardiovascular disease among the elderly study participants.
Even as long as 12 years after the supplementation with Coenzyme Q10 and high selenium yeast, the elderly Swedish citizens who had gotten the active treatment rather than the placebo treatment in the KiSel-10 study showed significantly lower risk of death from heart disease. Moreover, the reduced risk in the active treatment group was seen in such subgroups of patients as those with diabetes, hypertension, ischemic heart disease, and impaired functional capacity. Professor Alehagen and his team of researchers have not determined all of the mechanisms of the protective action of the Coenzyme Q10 and selenium supplements, but they point to the documented effects of the supplement combination on heart function, oxidative stress, fibrosis, and inflammation. Alehagen, U., Alexander, J., Aaseth, J. & Larsson, A. (2019). Decrease in inflammatory biomarker concentration by intervention with selenium and Coenzyme Q10: a sub-analysis of osteopontin, osteoprotergerin, TNFr1, TNFr2, and TWEAK. Journal of Inflammation, 16(5), 1-9. Professor Alehagen and his colleagues sought to explain the mechanism by which daily combined supplementation for four years with 200 micrograms of an organic high-selenium yeast preparation (SelenoPrecise®) and 200 milligrams of Coenzyme Q10 (Bio-Quinone Q10 100 mg twice daily) significantly reduced cardiovascular mortality and improved heart function in senior citizens aged 70-88 years at baseline. One plausible explanation is that the combined supplementation is associated with a significant reduction in numerous bio-markers of inflammation. Inflammation is a prominent feature in the pathogenesis of many diseases. First, in 2015, Professor Alehagen was able to demonstrate that the combined supplementation was singificantly associated with positive changes in the two bio-markers for inflammation C-reactive protein and sP-selectin, indicating a mechanism for reduced inflammation and atherosclerosis. Now, in this 2019 publication, Professor Alehagen reports significant reductions in four of five additional plasma bio-markers for inflammation: osteopontin, osteoprotegerin, sTNF receptor 1, and sTNF receptor 2. The only plasma bio-marker that did not show a significant reduction associated with the selenium yeast and Coenzyme Q10 supplementation was TWEAK (the tumor necrosis factor-like weak inducer of apoptosis). Conclusion: The senior citizens enrolled in the KiSel-10 study had sub-optimal intakes of selenium and sub-optimal bio-synthesis of Coenzyme Q10. Consequently, the combined supplementation improved their defenses against inflammation and oxidative stress and resulted in improved health-related quality of life, improved heart function, and reduced cardiovascular mortality. |
| Researcher | Beatrice Golomb |
| RESEARCHER AFFILIATION | Department of Medicine, University of California, San Diego, La Jolla, CA, USA |
| PUBLICATION CITATION | Golomb, B. A., Allison, M., Koperski, S., Koslik, H. J., Devaraj, S., & Ritchie, J. B. (2014). Coenzyme Q10 benefits symptoms in Gulf War veterans: results of a randomized double-blind study. Neural Computation, 26(11), 2594-2651. |
| TYPE OF STUDY | 3.5 ± 0.5 month-long randomized, double-blind, placebo-controlled clinical trial |
| SAMPLE SIZE | 46 Gulf War veterans meeting Kansas and Centers for Disease Control criteria for Gulf War illness |
| DOSAGE | Bio-Quinone Q10 100 mg per day or 300 mg per day |
| RESULTS | |
|
Supplementation with 100 mg and 300 mg of Q10 per day yielded improvement in physical function and symptoms in veterans with Gulf War illness. Among males (85% of enrollees), there were statistically significant benefits from 100 mg/day of Bio-Quinone Q10 on General Self-Rated Health and on physical function scores In 19 of 20 symptoms (sleep problems being the exception), Q10 supplementation was associated with signs of improvement, with several of the symptoms showing statistically significant improvement. |
| Researcher | Hanna Brauner, Annelie Brauner |
| RESEARCHER AFFILIATION | Department of Microbiology, Tumor and Cell Biology, Karolinska Institut, Stockholm, Sweden |
| PUBLICATION CITATION | Brauner, H., Lüthje, P., Grünler, J., Ekberg, N. R., Dallner, G., Brismar, K., & Brauner, A. (2014). Markers of innate immune activity in patients with type 1 and type 2 diabetes mellitus and the effect of the anti-oxidant coenzyme Q10 on inflammatory activity. Clinical and Experimental Immunology, 177(2), 478-482 |
| TYPE OF STUDY | Placebo-controlled study |
| SAMPLE SIZE | 58 diabetes patients, 27 with Type 1 Diabetes mellitus and 31 with Type 2 Diabetes mellitus, and also 19 healthy controls |
| DOSAGE | Bio-Quinone Q10 100 mg twice daily for 12 weeks |
| RESULTS | |
|
The researchers’ data suggest that Q10 supplementation can boost the immune system in Type 1 diabetics. Supplementation with Coenzyme Q10 reduces diabetes-associated inflammatory processes. Furthermore, Q10 supplementation may help prevent late complications. The researchers observed signs of reduced inflammation, increased cytokine production capacity, improved NK cell activity, and reduced hBD2 expression in Type 1 diabetics receiving daily Q10 supplements. (hBD2 expression is indicative of pro-inflammatory activity) |
| Researcher | Mario D. Cordero |
| RESEARCHER AFFILIATION | Facultad de Medicina, Universidad de Sevilla, Sevilla, Spain |
| PUBLICATION CITATION | Cordero, M. D., Alcocer-Gómez, E., Culic, O., Carrión, A. M., de Miguel, M., Díaz-Parrado, E., & Sánchez-Alcazar, J. A. (2014). NLRP3 inflammasome is activated in fibromyalgia: the effect of coenzyme Q10. Antioxidants & Redox Signaling, 20(8), 1169-1180. |
| TYPE OF STUDY | 40-day randomized, double-blind, placebo-controlled study |
| SAMPLE SIZE | 20 adult fibromyalgia patients |
| DOSAGE | Bio-Quinone Q10 300 mg/day (100 mg three times a day) |
| RESULTS | |
|
The researchers investigated the association between Coenzyme Q10 deficiency in fibromyalgia patients and reduced NLRP3 inflammasome activation and IL-1β and IL-18 serum levels. Their results show an important role for Q10 in the control of inflammasome (inflammasome = a multi-protein complex responsible for activation of inflammatory processes). Coenzyme Q10 supplementation at the level of 300 mg/day represents a new therapeutic intervention for fibromyalgia. |
| Researcher | Elisabet Alcocer-Gomez |
| RESEARCHER AFFILIATION | Oral Medicine Department, University of Sevilla, Sevilla, Spain |
| PUBLICATION CITATION | Alcocer-Gómez, E., Culic, O., Navarro-Pando, J. M., Sánchez-Alcázar, J. A., & Bullón, P. (2017). Effect of Coenzyme Q10 on Psychopathological Symptoms in Fibromyalgia Patients. CNS Neuroscience & Therapeutics, 23(2), 188–189. https://doi-org.db14.linccweb.org/10.1111/cns.12668 |
| TYPE OF STUDY | 40-day randomized, double-blind, placebo-controlled clinical trial |
| SAMPLE SIZE | 20 adult fibromyalgia patients |
| DOSAGE | Bio-Quinone Q10 300 mg/day (100 mg three times a day) |
| RESULTS | |
|
CoQ10 treatment induced important molecular and clinical improvements: |
| Researcher | Jan Fedacko |
| RESEARCHER AFFILIATION | Pavol Jozef Safarik University, Kosice, Slovakia |
| PUBLICATION CITATION | Fedacko, J., Pella, D., Fedackova, P., Hänninen, O., Tuomainen, P., Jarcuska, P., & ... Littarru, G. P. (2013). Coenzyme Q10 and selenium in statin-associated myopathy treatment. Canadian Journal of Physiology and Pharmacology, 91(2), 165-170. |
| TYPE OF STUDY | Randomized, double-blind, placebo-controlled clinical trial |
| SAMPLE SIZE | 60 patients reporting statin-associated myopathy (muscle pain, muscle weakness, tiredness, or muscle cramps) |
| DOSAGE | Group 1. Bio-Quinone Q10 200 mg + 200 ug of selenium daily Group 2. Bio-Quinone Q10 200 mg + a selenium placebo daily Group 3. 200 ug of selenium + Q10 placebo daily Group 4. Q10 placebo + selenium placebo |
| RESULTS | |
|
In patients treated with Bio-Quinone Q10, the researchers observed a decrease in the symptoms of statin-associated myopathy, both in the numbers of the symptoms and in the intensity of the symptoms. Compared with placebo, the Q10 supplementation produced a reduction of muscle pain and muscle weakness and fatigue. |
| Researcher | Martin Bogsrud |
| RESEARCHER AFFILIATION | Ålesund Hospital, Ålesund, Norway |
| PUBLICATION CITATION | Bogsrud, M. P., Langslet, G., Ose, L., Arnesen, K., Sm Stuen, M. C., Malt, U. F., & ... Retterstøl, K. (2013). No effect of combined coenzyme Q10 and selenium supplementation on atorvastatin-induced myopathy. Scandinavian Cardiovascular Journal, 47(2), 80-87. |
| TYPE OF STUDY | 12-week randomized, double-blind, placebo-controlled trial |
| SAMPLE SIZE | 43 eligible patients reporting statin-associated myopathy (muscle pain, weakness) |
| DOSAGE | Bio-Quinone Q10 (Myoquinon) 400 mg/day and SelenoPrecise 200 micrograms/day |
| RESULTS | |
|
The administration of the statin medication atorvastatin reduced significantly the patients’ blood Coenzyme Q10 levels in both groups. Supplementation with Myoquinon then significantly increased CoQ10 levels in the active treatment group. |
| Researcher | Amar Singh Thakur |
| RESEARCHER AFFILIATION | Department of Biochemistry, Government Medical College, Jagdalpur, India |
| PUBLICATION CITATION | Thakur, A. S., Littarru, G. P., Moesgaard, S., Dan Sindberg, C., Khan, Y., & Singh, C. M. (2013). Hematological Parameters and RBC TBARS Level of Q10 Supplemented Tribal Sickle Cell Patients: A Hospital Based Study. Indian Journal of Clinical Biochemistry: IJCB, 28(2), 185-188 |
| TYPE OF STUDY | 6-month placebo-controlled trial |
| SAMPLE SIZE | 34 patients aged 10 to 55 years |
| DOSAGE | Bio-Quinone Q10 200 mg daily |
| RESULTS | |
|
With Q10 supplementation, changes in hematological parameters were observed: |
| Researcher | Luca Tiano |
| RESEARCHER AFFILIATION | Institute of Biochemistry, Polytechnic University of the Marche, Ancona, Italy |
| PUBLICATION CITATION | Tiano, L., Carnevali, P., Padella, L., Santoro, L., Principi, F., Brugè, F., & Littarru, G. P. (2011). Effect of Coenzyme Q10 in mitigating oxidative DNA damage in Down syndrome patients, a double blind randomized controlled trial. Neurobiology of Aging, 32(11), 2103-2105. Tiano, L., Padella, L., Santoro, L., Carnevali, P., Principi, F., Brugè, F., & ... Littarru, G. P. (2012). Prolonged coenzyme Q10 treatment in Down syndrome patients: effect on DNA oxidation. Neurobiology of Aging, 33(3), 626.e1-8. |
| TYPE OF STUDY | Randomized, double-blind, placebo-controlled trial |
| SAMPLE SIZE | 30 Down syndrome patients and 30 healthy control patients, aged 4 – 12 years |
| DOSAGE | Bio-Quinone Q10 4 mg/kg per day |
| RESULTS | |
|
The purpose of the study was to investigate the efficacy of Q10 supplementation in blocking the effect of oxidative damage in Down syndrome patients. The earlier shorter study (6 months) demonstrated a mild protective effect of Q10 on DNA, and the Q10 treatment did seem to attenuate oxidative damage. In the continuation of the trial, the results indicated an age-specific reduction in the percentage of cells showing the highest amount of oxidation. Overall, the researchers concluded that there is a potential role of Q10 treatment in modulating DNA repair mechanisms. |
| Researcher | Christopher F. Spurney |
| RESEARCHER AFFILIATION | George Washington University, Washington D. C. |
| PUBLICATION CITATION | Spurney, C. F., Rocha, C. T., Henricson, E., Florence, J., Mayhew, J., Gorni, K., & Escolar, D. M. (2011). CINRG pilot trial of coenzyme Q10 in steroid-treated Duchenne muscular dystrophy. Muscle & Nerve, 44(2), 174–178. https://doi-org.db14.linccweb.org/10.1002/mus.22047 |
| TYPE OF STUDY | Open label pilot study |
| SAMPLE SIZE | 12 children aged 5 – 10 years diagnosed with Duchenne Muscular Dystrophy and prescribed prednisone corticosteroid treatment |
| DOSAGE | Bio-Quinone Q10 90 mg/day initially and increasing by 30 mg/day increments until the children's serum CoQ10 levels reached 2.5 mcg/ml |
| RESULTS | |
|
Nine of the 12 children achieved an increase in their total Quantitative Muscle Testing scores. Overall, the Bio-Quinone CoQ10 treatment resulted in an 8.5% increase in muscle strength. The addition of daily CoQ10 treatment to the prednisone therapy resulted in an increase in muscle strength in the Duchenne muscular dystrophy patients. |
| Researcher | Giancarlo Balercia |
| RESEARCHER AFFILIATION | School of Medicine, University of Ancona, Ancona, Italy |
| PUBLICATION CITATION | Balercia, G., Buldreghini, E., Vignini, A., Tiano, L., Paggi, F., Amoroso, S., & Littarru, G. (2009). Coenzyme Q10 treatment in infertile men with idiopathic asthenozoospermia: a placebo-controlled, double-blind randomized trial. Fertility and Sterility, 91(5), 1785-1792. |
| TYPE OF STUDY | 6-month-long randomized, double-blind, placebo-controlled clinical trial |
| SAMPLE SIZE | 60 infertile patients (27-39 years of age) with low sperm motility |
| DOSAGE | Bio-Quinone Q10 200 mg/day, 100 mg twice daily orally for 6 months |
| RESULTS | |
|
Treatment significantly increased both ubiquinone and ubiquinol levels in seminal plasma and sperm cells. Treatment also increased spermatozoa motility significantly. Q10 supplementation proved to be effective at improving sperm motility in patients diagnosed with idiopathic asthenozoospermia (reduced sperm motility of unknown cause). |
| Researcher | Niels Hertz |
| RESEARCHER AFFILIATION | Private practice, Vipperød, Denmark |
| PUBLICATION CITATION | Hertz, N., & Lister, R. E. (2009). Improved survival in patients with end-stage cancer treated with coenzyme Q10 and other antioxidants: a pilot study. The Journal of International Medical Research, 37(6), 1961-1971. |
| TYPE OF STUDY | 9-year open-label pilot study |
| SAMPLE SIZE | 41 end-stage cancer patients (with varying diagnoses) |
| DOSAGE | Bio-Quinone Q10 in varying amounts plus a mixture of other antioxidants (vitamin C, selenium, folic acid, and beta-carotene) |
| RESULTS | |
|
Over a period of 9 years, the attending physician followed 41 patients with end-stage cancer until death. The primary cancers were, variously, in the breast, brain, lungs, kidneys, pancreas, esophagus, stomach, colon, prostate, ovaries, and skin. The patients’ median predicted survival time was 12 months (range 3 - 29 months), and the actual median survival, with Q10 and antioxidant supplementation, was 17 months (1 - 120 months), 40% longer than the median predicted survival. |
| Researcher | J. M. Cooper |
| RESEARCHER AFFILIATION | University Department of Clinical Neurosciences, Institute of Neurology, University College London, London, UK |
| PUBLICATION CITATION | Cooper, J. M., Korlipara, L. P., Hart, P. E., Bradley, J. L., & Schapira, A. V. (2008). Coenzyme Q10 and Vitamin E deficiency in Friedreich's ataxia: predictor of efficacy of vitamin E and coenzyme Q10 therapy. European Journal of Neurology: The Official Journal of the European Federation of Neurological Societies, 15(12), 1371-1379. |
| TYPE OF STUDY | 2-year randomized, double-blind, placebo-controlled clinical trial |
| SAMPLE SIZE | 50 Friedreich’s ataxia patients (Friedreich’s ataxia is a rare inherited disease causing problems in the nervous system damage and in movement) |
| DOSAGE | High-dose group: Bio-Quinone Q10 600 mg/day (2 * 100 mg three times a day) and Vitamin E 2100 IU/day (2 * 525 IU twice a day); Patients under 18 years: Bio-Quinone Q10 9 mg/kg/day and Vitamin E 30 IU/kg/day; Low-dose group: Bio-Quinone Q10 30 mg and placebo tablets |
| RESULTS | |
|
The Friedreich’s ataxia patients had low serum Q10 and Vitamin E at baseline. Supplementation increased serum levels of Q10 and Vitamin E significantly. Both the low-dose and the high-dose Q10 treatments were effective in improving ICARS scores.
|
| Researcher | Giovanni Montini |
| RESEARCHER AFFILIATION | Pediatric Department, University Hospital, Padua, Italy |
| PUBLICATION CITATION | Montini, G., Malaventura, C., & Salviati, L. (2008). Early Coenzyme Q10 supplementation in primary coenzyme Q10 deficiency. The New England Journal of Medicine, 358(26), 2849-2850. |
| TYPE OF STUDY | Case reports |
| SAMPLE SIZE | 2 very young children, siblings, diagnosed with Coenzyme Q10 deficiency |
| DOSAGE | Bio-Quinone Q10 30 mg per kilogram of body weight per day |
| RESULTS | |
|
The disease condition Primary Coenzyme Q10 Deficiency manifests itself in nephropathy (diminished kidney function) and in encephalomyopathy (disorder of the brain and spinal system). In patient number one, administration of Q10 resulted in neurological improvement but no change in the status of renal function. In patient number two, after 20 days of the administration of Q10, the researchers observed progressive recovery of renal function. They concluded that early administration of Coenzyme Q10 was important for the resolution of renal symptoms and for the prevention of neurologic damage in patient number two. |
| Researcher | Hilde Grindvik Nielsen |
| RESEARCHER AFFILIATION | Center for Clinical Research, Ullevaal University Hospital, University of Oslo, Norway |
| PUBLICATION CITATION | Nielsen, H. G., Skjønsberg, O. H., & Lyberg, T. (2008). Effect of antioxidant supplementation on leucocyte expression of reactive oxygen species in athletes. Scandinavian Journal of Clinical and Laboratory Investigation, 68(7), 526-533. |
| TYPE OF STUDY | 4-week randomized, double-blind, placebo-controlled cross-over study |
| SAMPLE SIZE | 18 well-trained endurance athletes, 14 males with a mean age of 28 years and 4 females with a mean age of 24 years |
| DOSAGE | Bio-Quinone Q10 200 mg and Bio-Antioxidant (with 400 mg vitamin C and 180 mg vitamin E), both taken as one dose daily |
| RESULTS | |
|
The researchers found, after four weeks of antioxidant supplementation, no significant differences in the intracellular levels of reactive oxygen species (ROS) in the athletes’ leucocytes (white blood cells). |
| Researcher | Andrejs Skesters |
| RESEARCHER AFFILIATION | Riga Stradin University, Riga, Latvia |
| PUBLICATION CITATION | Skesters, A., Zvagule, T., Larmane, L., Rainsford, K., Silova, A., Rusakova, N., Mustafins, P. (2008). Effects of selenium alone and with antioxidants and ibuprofen mixture in Chernobyl catastrophe clean-up workers at risk of developing cancer. Cell Biology and Toxicology, 24(1), S31. |
| TYPE OF STUDY | 1-year randomized, double-blind, placebo-controlled study |
| SAMPLE SIZE | 134 Chernobyl catastrophe clean-up workers at risk of developing cancer, aged 43–55 years |
| DOSAGE | Three groups: 200 micrograms Selenium yeast alone 200 micrograms Selenium yeast in combination with 100 mg Bio-Quinone Q10, additional antioxidants, and ibuprofen Placebo |
| RESULTS | |
|
Increased concentrations of selenium, vitamin E, and total antioxidant status were associated positively with the following outcomes: |
| Researcher | Katarina Skough |
| RESEARCHER AFFILIATION | Division of Rehabilitation Medicine, Department of Clinical Sciences, Danderyds Hospital, Stockholm, Sweden |
| PUBLICATION CITATION | Skough, K., Krossén, C., Heiwe, S., Theorell, H., & Borg, K. (2008). Effects of resistance training in combination with coenzyme Q10 supplementation in patients with post-polio: a pilot study. Journal of Rehabilitation Medicine, 40(9), 773-775. |
| TYPE OF STUDY | 12-week parallel randomized, double-blind placebo-controlled, pilot study. |
| SAMPLE SIZE | 14 patients (8 women and 6 men) with post-polio syndrome |
| DOSAGE | Q10 200 mg/day |
| RESULTS | |
|
All 14 patients took part in a resistance training program 3 days per week for 12 weeks. All 14 patients, those taking Q10 supplements and those taking a placebo, gained significantly in muscle strength, muscle endurance and quality of life. Taking the Q10 supplement did not confer any statistically significant strength and endurance benefits. Patients taking the Q10 supplements did record increases in the six-minute walk tests, which might possibly suggest an association between Q10 intake and muscular endurance. |
| Researcher | Pedro José Tauler Riera |
| RESEARCHER AFFILIATION | Universitat de les Illes Balears, Palma de Mallorca, Illes Balears, Spain |
| PUBLICATION CITATION | Tauler, P., Ferrer, M. D., Sureda, A., Pujol, P., Drobnic, F., Tur, J. A., & Pons, A. (2008). Supplementation with an antioxidant cocktail containing Coenzyme Q prevents plasma oxidative damage induced by soccer. European Journal of Applied Physiology, 104(5), 777-785. |
| TYPE OF STUDY | 3-month randomized, double-blind, placebo-controlled study |
| SAMPLE SIZE | 19 voluntary male pre-professional soccer players |
| DOSAGE | Bio-Quinone Q10 100 mg/day and Bio-Antioxidant |
| RESULTS | |
|
After 3 months of supplementation, which resulted in higher plasma levels of ascorbate and Coenzyme Q10 as compared to the placebo group levels, the subjects took part in a 60-minute soccer match. Following the match, the researchers found that the Q10 and antioxidant supplementation had influenced plasma oxidative stress markers positively. The levels of oxidative stress markers were lower in the supplemented group than in the placebo group following the match. The use of Q10 and antioxidant diet supplementation was seen to prevent plasma oxidative damage, but it did not influence the neutrophil response to the physical exertion of the soccer match. |
| Researcher | Tuomas Westermarck |
| RESEARCHER AFFILIATION | Rinnekoti Research Center, Espoo, Finland |
| PUBLICATION CITATION | Westermarck, T, Sauka, M., Selga, G., Skesters, A., & Abdulla, M., & Atroshi, F. (2008). Effects of cocktail antioxidant supplementation on oxidative stress in AIDS. Cell Biology and Toxicology, 24(1), S55-S56. |
| TYPE OF STUDY | 6-week double-blind, placebo-controlled clinical trial |
| SAMPLE SIZE | 24 Latvian male volunteers, HIV-infected outpatients, aged 35 years plus/minus 3 years and 10 uninfected control males similar in age to the HIV-infected volunteers |
| DOSAGE | Bio-Quinone Q10 100 mg per day and selenium 100 micrograms per day for 6 weeks |
| RESULTS | |
|
All patients in the study had low serum selenium (defined as a serum level ≤ 85 µg/l). Selenium deficiency correlates positively with the progression and mortality of HIV infections. Selenium is necessary for the proper functioning of the immune system. It seems to be a key nutrient in the inhibition of HIV infection to the development of AIDS. In this study, Q10 supplementation appeared to increase the serum selenium concentrations. Serum selenium levels increased following Q10 supplementation. |
| Researcher | Janko Žmitek |
| RESEARCHER AFFILIATION | National Institute of Chemistry, University of Ljubljana, Ljubljana, Slovenia |
| PUBLICATION CITATION | Zmitek, J., Smidovnik, A., Fir, M., Prosek, M., Zmitek, K., Walczak, J., & Pravst, I. (2008). Relative bioavailability of two forms of a novel water-soluble coenzyme Q10. Annals of Nutrition & Metabolism, 52(4), 281-287. |
| TYPE OF STUDY | Randomized three-period crossover clinical trial of two forms of a new water-soluble formulation compared to Bio-Quinone soft-gel capsules containing the Q10 in soybean oil |
| SAMPLE SIZE | 14 healthy non-smoking male volunteers aged 30–52 years with a body mass index between 20 and 25 |
| DOSAGE | Q10 60 mg single dose treatments (liquid, powder, and Bio-Quinone soft-gel formulations) |
| RESULTS | |
|
The Q10 plasma concentration curves for all three formulations were similar showing a peak concentration about 4 – 5 hours after ingestion. |
| Researcher | A. Lukmann |
| RESEARCHER AFFILIATION | University of Tartu, Estonia |
| PUBLICATION CITATION | Lukmann, A., Ojamaa, M., Veraksitch, A., Vihalemm, T., Zilmer, K., Zilmer, M. & Maaroos, J. (2007). The effects of Coenzyme Q10 in early rehabilitation after acute coronary syndrome. Kobe, Japan: The 5th Conference of the International Coenzyme Q10 Association: Program & Abstracts. |
| TYPE OF STUDY | 8-week randomized, double-blind, placebo-controlled clinical trial |
| SAMPLE SIZE | 58 acute coronary syndrome patients (31 patients received Bio-Quinone Q10; 27 patients received placebo) |
| DOSAGE | Bio-Quinone Q10 100/200 mg per day (1/7 weeks) |
| RESULTS | |
|
The administration of Bio-Quinone Q10 to acute coronary syndrome patients early in their rehabilitation was associated with a significant increase in most of the indices of cardiorespiratory reserve and functional capacity. There was a significant improvement in aerobic capacity in the Q10 group as compared to the placebo control group. |
| Researcher | Christian Sindberg |
| RESEARCHER AFFILIATION | Research Department, Pharma Nord, Vejle, Denmark |
| PUBLICATION CITATION | Sindberg, C. D., Littarru, G. P., Moesgaard,S., & Storm-Henningsen, P. L. (2007). Bioavailability of Coenzyme Q10 formulated with palm oil is equivalent with a similar soy oil formulation. Kobe, Japan: The 5th Conference of the International Coenzyme Q10 Association: Poster. |
| TYPE OF STUDY | 8-week randomized, double-blind, cross-over study |
| SAMPLE SIZE | 12 volunteers |
| DOSAGE | Two groups receiving Bio-Quinone Q10 preparations containing 100 mg Q10 and either 400 mg soy oil or 400 mg palm oil respectively each day for two periods of three weeks, with a two-week washout period in between |
| RESULTS | |
|
The statistical analysis was done using a paired t test. No adverse effects of taking either preparation were observed. |
| Researcher | Ram B. Singh |
| RESEARCHER AFFILIATION | Halberg Hospital and Research Institute, Moradabad, India |
| PUBLICATION CITATION | Singh, R. B., Niaz, M. A., Kumar, A., Sindberg, C. D., Moesgaard, S., & Littarru, G. P. (2005). Effect on absorption and oxidative stress of different oral Coenzyme Q10 dosages and intake strategy in healthy men. Biofactors (Oxford, England), 25(1-4), 219-224. |
| TYPE OF STUDY | 20-day randomized, double-blind, placebo-controlled clinical trial |
| SAMPLE SIZE | 60 healthy men, aged 18-55 years |
| DOSAGE | Various dosages and dose strategies of coenzyme Q10 soft oil capsules (Myoqinon 100 mg) or crystalline 100 mg Q10 powder capsules or placebo |
| RESULTS | |
|
Patient compliance (checked by capsule counting) was above 90%. The side effects of taking Coenzyme Q10 supplements were negligible. Supplementation increased the patients’ serum Q10 levels significantly 3 - 10 fold. The Coenzyme Q10 dissolved in oil was absorbed more effectively than the same amount of crystal powder Q10 in raising serum Q10 levels. A divided dose strategy – 100 mg twice a day – gave a better serum Q10 response than a single daily dose of 200 mg. Supplementation with 200 mg of Coenzyme Q10 for 20 days resulted in significantly reduced levels of malondialdehyde, a biological marker for oxidative stress. The Indian patients had generally lower baseline serum Q10 levels, possibly because of their vegetarian diets. |
| Researcher | J. Soongswang |
| RESEARCHER AFFILIATION | Division of Cardiology, Department of Pediatrics, Faculty of Medicine, Siriraj Hospital, Mahidol University, Bangkok, Thailand. |
| PUBLICATION CITATION | Soongswang, J., Sangtawesin, C., Durongpisitkul, K., Laohaprasitiporn, D., Nana, A., Punlee, K., & Kangkagate, C. (2005). The effect of coenzyme Q10 on idiopathic chronic dilated cardiomyopathy in children. Pediatric Cardiology, 26(4), 361-366. |
| TYPE OF STUDY | 9-month open-label prospective study |
| SAMPLE SIZE | 15 patients diagnosed with idiopathic chronic dilated cardiomyopathy with a median age of 4.4 years (range, 0.6-16.3). |
| DOSAGE | Bio-Quinone Q10 3.1 mg/kg/day for 9 months as a supplement to a fixed amount of conventional anti-failure drugs |
| RESULTS | |
|
Patients receiving Q10 supplementation for 9 months did not show significant improvement in such parameters as weight, growth rate, cardio-thoracic ratio (CT ratio is an indirect measure of heart size), heart rate, or left ventricle ejection fraction. However, 4 of the 15 patients receiving the Q10 adjunctive treatment did improve one NYHA functional class, and one patient improved two NYHA functional classes. The researchers concluded that Q10 supplementation may be a good non-invasive manner to improve NYHA functional class, admission, and ventricular depolarization, but, in the present study, it did not seem to help overall cardiac function. |
| Researcher | Paul E. Hart |
| RESEARCHER AFFILIATION | University Department of Clinical Neurosciences, Royal Free and University College Medical School, London, England |
| PUBLICATION CITATION | Hart, P. E., Lodi, R., Rajagopalan, B., Bradley, J. L., Crilley, J. G., Turner, C., & Cooper, J. M. (2005). Antioxidant treatment of patients with Friedreich ataxia: four-year follow-up. Archives of Neurology, 62(4), 621-626. |
| TYPE OF STUDY | Open-label pilot study over 47 months |
| SAMPLE SIZE | 77 patients with clinically and genetically defined Friedreich’s ataxia |
| DOSAGE | Bio-Quinone Q10 400 mg/day (2 times 100 mg twice a day) and Vitamin E 2100 international units/day (2 times 525 international units twice a day) |
| RESULTS | |
|
The researchers observed significant improvement in cardiac and skeletal muscle bioenergetics throughout the 47 months of therapy. Echocardiographic data revealed significantly increased fractional shortening (a measure of the pumping function of the heart) at the both the 35-month and the 47-month time points. The researchers concluded that Q10 and Vitamin E resulted in a sustained improvement in mitochondrial energy synthesis that was associated with a slowing of the progression of certain clinical features of the disease and with a significant improvement in cardiac function. (See Cooper 2008 for the results of a randomized, double-blind, placebo-controlled study involving Q10 supplementation of Friedreich’s ataxia patients.) |
| Researcher | K. F. Hoenjet |
| RESEARCHER AFFILIATION | Department of Urology, University Hospital of Maastricht, Maastricht, The Netherlands |
| PUBLICATION CITATION | Hoenjet, K. F., Dagnelie, P. C., Delaere, K. J., Wijckmans, N. G., Zambon, J. V., & Oosterhof, G. N. (2005). Effect of a nutritional supplement containing vitamin E, selenium, vitamin c and coenzyme Q10 on serum PSA in patients with hormonally untreated carcinoma of the prostate: a randomised placebo-controlled study. European Urology, 47(4), 433-439. |
| TYPE OF STUDY | 21-week randomized, double-blind, placebo-controlled study |
| SAMPLE SIZE | 80 patients with hormonally untreated carcinoma of the prostate and rising serum PSA levels |
| DOSAGE | Q10 200 mg per day (2 times 100 mg) |
| RESULTS | |
|
The serum Q10 level increased from 1.42 mg/L (1.13 – 1.77mg/L) before treatment to 2.63 mg/L (2.15 – 3.11 mg/L) following treatment. In the treatment group, supplementation raised the serum levels of Q10, vitamin E, and selenium significantly over the 21-week study period. The researchers did not observe any significant differences between the treatment group and placebo group in the serum levels of PSA, testosterone, di-hydrotestosterone, luteinizing hormone, or sex hormone binding globulin. |
| Researcher | Giancarlo Balercia |
| RESEARCHER AFFILIATION | Department of Internal Medicine, School of Medicine, University of Ancona, Ancona, Italy |
| PUBLICATION CITATION | Balercia, G., Mosca, F., Mantero, F., Boscaro, M., Mancini, A., Ricciardo-Lamonica, G., & Littarru, G. (2004). Coenzyme Q10 supplementation in infertile men with idiopathic asthenozoospermia: an open, uncontrolled pilot study. Fertility and Sterility, 81(1), 93-98. |
| TYPE OF STUDY | 6-month open, uncontrolled pilot study |
| SAMPLE SIZE | 22 patients (mean age, 31 years; range, 25–39 years) with idiopathic asthenozoospermia (reduced sperm motility) |
| DOSAGE | Bio-Quinone Q10 200 mg/day (100 mg twice daily for 6 months) |
| RESULTS | |
|
The researchers’ analysis confirmed a significant increase in sperm cell motility as well. Supplementation with Q10 should be considered as a treatment option in cases of asthenozoospermia. |
| Researcher | Eva Rabing Christensen |
| RESEARCHER AFFILIATION | Department of Infectious Diseases, Skejby Sygehus, Aarhus University Hospital, Aarhus, Denmark. |
| PUBLICATION CITATION | Rabing Christensen, E., Stegger, M., Jensen-Fangel, S., Laursen, A. L., & Ostergaard, L. (2004). Mitochondrial DNA levels in fat and blood cells from patients with lipodystrophy or peripheral neuropathy and the effect of 90 days of high-dose coenzyme Q treatment: a randomized, double-blind, placebo-controlled pilot study. Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America, 39(9), 1371-1379. |
| TYPE OF STUDY | 3-month randomized, double-blind, placebo-controlled trial |
| SAMPLE SIZE | 25 HIV-infected patients and 10 healthy controls |
| DOSAGE | Bio-Quinone Q10 200 mg (2 times 100 mg daily, at breakfast and at dinner) |
| RESULTS | |
|
The Coenzyme Q10 therapy significantly improved the general condition and well-being of the asymptomatic HIV-infected patients and caused a reversible increase in peripheral neuropathy pain (P=.048). At the same time, the Q10 supplementation seemed to aggravate pain in patients with peripheral neuropathy. |
| Researcher | Cestimir Zita |
| RESEARCHER AFFILIATION | Medical Faculty Hospital, Prague, Czech Republic |
| PUBLICATION CITATION | Zita, C., Overvad, K., Mortensen, S. A., Sindberg, C. D., Moesgaard, S., & Hunter, D. A. (2003). Serum coenzyme Q10 concentrations in healthy men supplemented with 30 mg or 100 mg coenzyme Q10 for two months in a randomised controlled study. Biofactors (Oxford, England), 18(1-4), 185-193. |
| TYPE OF STUDY | Two-month randomized, double-blind, placebo-controlled study |
| SAMPLE SIZE | 99 men with a median baseline serum Q10 level of 1.26 mg/liter (10%, 90% fractiles: 0.82, 1.83) |
| DOSAGE | Bio-Quinone Q10 30 mg or 100 mg once per day for two months |
| RESULTS | |
|
From author abstract: Supplementation with 30 mg or 100 mg Q10 resulted in median increases in serum Q10 concentration of 0.55 mg/l and 1.36 mg/l, respectively, compared with a median decrease of 0.23 mg/l with placebo. The changes in the Q10 groups were significantly different from those in the placebo group, and the increase in the 100 mg Q10 group was significantly greater than that in the 30 mg Q10 group. The change in serum Q10 concentration in the Q10 groups did not depend on baseline serum Q10 concentration, age, or body weight. |
|
| Researcher | Huda Elshershari |
| RESEARCHER AFFILIATION | Department of Pediatric Cardiology, Hacettepe University Children s Hospital, Ankara, Turkey |
| PUBLICATION CITATION | Elshershari, H., Ozer, S., Ozkutlu, S., & Ozme, S. (2003). Potential usefulness of Coenzyme Q10 in the treatment of idiopathic dilated cardiomyopathy in children. International Journal of Cardiology, 88(1), 101-102. |
| TYPE OF STUDY | Open-label study lasting a mean period of 8 months plus/minus 6 months |
| SAMPLE SIZE | 6 children ranging in age from 2 months to 11 years, each child) with idiopathic dilated cardiomyopathy and a clinical picture of congestive heart failure |
| DOSAGE | Bio-Quinone Q10 in a dosage of 10 mg/kg per day in divided 2 or 3 daily doses |
| RESULTS | |
|
The researchers continued the conventional therapy of digitalis, diuretics, and angiotensin-converting-enzyme inhibitors during the period of the Bio-Quinone Q10 supplementation. In five of the six cases, there was significant increase in ejection fraction (more blood pumped from the heart with each heartbeat) and significant increase in fractional shortening (the increase indicating better heart muscle contractions). In the case of the sixth child, there was no observed improvement in myocardial function, but there was an improvement from NYHA class III to class II. |
| Researcher | Martin W. I. M. Horstink |
| RESEARCHER AFFILIATION | University Hospital Nijmegen, The Netherlands |
| PUBLICATION CITATION | Horstink, M. M., & van Engelen, B. G. (2003). The effect of coenzyme Q10 therapy in Parkinson disease could be symptomatic. Archives of Neurology, 60(8), 1170-1172. |
| TYPE OF STUDY | 6-month open-label pilot study |
| SAMPLE SIZE | 12 patients with idiopathic Parkinson disease, ranging in age from 56 to 67 years and all without signs of dementia |
| DOSAGE | Bio-Quinone Q10 1000 mg per day (500 mg twice daily) for three months and then Bio-Quinone Q10 1500 mg per day (500 mg three times daily) for three months |
| RESULTS | |
|
Most variables did not show improvement with Q10 treatment, but the sum score used in the study did show an improvement in motor performance as a result of the increase to 1500 mg of Q10 per day. However, clinical effects in the patients were minor. The 1000 mg/day treatment with Bio-Quinone Q10 did produce higher plasma Q10 levels than did treatment with a different Q10 preparation used in the trial reported in Shults, C. W., Oakes, D., Kieburtz, K., Beal, M. F., Haas, R., Plumb, S., & ... Lew, M. (2002). Effects of coenzyme Q10 in early Parkinson disease: evidence of slowing of the functional decline. Archives of Neurology, 59(10), 1541-1550. |
| Researcher | V. Z. Lankin |
| RESEARCHER AFFILIATION | Cardiology Research Complex, Miasnikov's Institute of Clinical Cardiology, Moscow, Russia |
| PUBLICATION CITATION | Lankin, V. Z., Tikhaze, A. K., Kukharchuk, V. V., Konovalova, G. G., Pisarenko, O. I., Kaminnyi, A. I., & ... Belenkov, Y. N. (2003). Antioxidants decrease the intensification of low density lipoprotein in vivo peroxidation during therapy with statins. Molecular and Cellular Biochemistry, 249(1-2), 129-140. |
| TYPE OF STUDY | 6-month randomized, double-blind, placebo-controlled clinical trial |
| SAMPLE SIZE | First study: 20 patients (49 years old +/- 2.5 years) with chronic coronary heart disease who were taking pravastatin Second study: 32 patients (53 years old +/- 5 years) with chronic coronary heart disease who were taking cerivastatin |
| DOSAGE | Bio-Quinone Q10 60 mg per day |
| RESULTS | |
|
Six months of therapy with 60 mg of Q10 daily together with the conventional treatment with the HMG-CoA reductase inhibitor pravastatin led to a significant decrease in the lipoperoxide level in patients’ LDL lipoproteins. The researchers concluded that Q10 administered in the form of ubiquinone may be effective in the prevention of atherogenic oxidative damage of LDL during statin therapy. The findings in this study indicate that the long-term use of statins suppresses Coenzyme Q10 bio-synthesis in heart muscle cells and impairs the supply of energy to the heart. |
| Researcher | Hilde Grindvik Nielsen |
| RESEARCHER AFFILIATION | Center for Clinical Research, Ullevaal University Hospital, Oslo, Norway |
| PUBLICATION CITATION | Nielsen, H. G., Saetre, L., Skjønsberg, O. H., & Lyberg, T. (2003). Antioxidant supplementation and leucocyte expression of reactive oxygen species (ROS) in endurance-trained athletes. Copenhagen, Denmark: International Society for Exercise and Immunology Symposium. |
| TYPE OF STUDY | 4-week randomized, double-blind, placebo-controlled cross-over study |
| SAMPLE SIZE | 12 male athletes with a mean age of 27 years and 4 female athletes with a mean age of 24 years |
| DOSAGE | Bio-Quinone Q10 200 mg and Bio-Antioxidant (with 400 mg vitamin C and 180 mg vitamin E), both taken as one dose daily |
| RESULTS | |
|
4 weeks of antioxidant supplementation was not associated with any significant differences in the leukocyte reactive oxygen species (ROS) levels in the well-trained endurance athletes. One possible explanation might be that the sample size in this study was small, and the intervention period was short. There was perhaps insufficient time for the total plasma antioxidant status to improve. |
| Researcher | Jytte Engelsen, Jørn Dalsgaard Nielsen & Kaj Flemming Winther Hansen |
| RESEARCHER AFFILIATION | Amtssygehuset i Gentofte, (County Hospital in Gentofte), Hellerup, Denmark |
| PUBLICATION CITATION | Engelsen, J., Nielsen, J. D., & Winther, K. (2002). Effect of coenzyme Q10 and Ginkgo biloba on warfarin dosage in stable, long-term warfarin treated outpatients. A randomised, double blind, placebo-crossover trial. Thrombosis and Haemostasis, 87(6), 1075-1076. Also reported in Ugeskrift for Læger 2003; 165(18):1868-71. |
| TYPE OF STUDY | Randomized, double-blind, placebo-controlled crossover trial |
| SAMPLE SIZE | 24 stable, long-term warfarin-treated outpatients |
| DOSAGE | Bio-Quinone Q10 100 mg per day and Bio-Biloba 100 mg Ginkgo biloba extract per day each given in random order over treatment periods of four weeks with each period followed by a two week wash out period |
| RESULTS | |
|
The researchers found a reduced response to warfarin by patients taking the Q10 supplement and an increased response to warfarin by the patients taking Gingko biloba extract. The patients’ INR (international normalized ratio) was stable during the entire treatment period. The researchers concluded that Coenzyme Q10 and Ginkgo biloba do not influence the clinical effect of warfarin. |
| Researcher | Robert Eric Lister |
| RESEARCHER AFFILIATION | Phylax Ltd, Beaconsfield, UK |
| PUBLICATION CITATION | Lister, R. E. (2002). An open pilot study to evaluate the potential benefits of coenzyme Q10 combined with Ginkgo biloba extract in fibromyalgia syndrome. The Journal of International Medical Research, 30(2), 195-199. |
| TYPE OF STUDY | Open-label pilot study |
| SAMPLE SIZE | 25 volunteer subjects, comprising both sexes, all diagnosed with fibromyalgia |
| DOSAGE | Bio-Quinone Q10 200 mg Q10 daily and 200 mg Ginkgo biloba extract daily for 84 days |
| RESULTS | |
|
68 per cent of the patients completing the treatment indicated at the end of the study that they would like to continue with the treatment. The researchers measured the patients’ quality of life at 0-, 4-, 8-, and 12-week intervals using the Dartmouth Primary Care Cooperative Information Project/World Organization of Family Doctors (COOP/WONCA) questionnaire. The patients’ scores on the measure got progressively better, and the scores at the end of the study were significantly better than the scores had been at the start of the study. |
|
| Researcher | S. J. Hodges |
| RESEARCHER AFFILIATION | Institute of Hepatology, Department of Medicine, UCL, London, UK |
| PUBLICATION CITATION | Hodges, S. J., Gill, K., Walsh, T., Lunn, R., & Rawlinson, A. (2002). Human gingival crevicular fluid levels of Coenzyme Q10. London, UK: Third conference of the International Coenzyme Q10 Association. 77-78. |
| TYPE OF STUDY | Open label pilot study |
| SAMPLE SIZE | 9 patients (6 female, 3 male), mean age of 41 years, each patient with one healthy and one diseased periodontal site compared with 15 healthy volunteers |
| DOSAGE | Bio-Quinone Q10 30 mg, 60 mg, and 120 mg single dose |
| RESULTS | |
|
The primary focus of the study was on the efficacy of Q10 toothpaste in delivering Q10 to gingival crevicular fluid. The administration of 120 mg of Bio-Quinone Q10 as a single dose to a 55-year-old volunteer did result in detection of Q10 in the gingival crevicular fluid. The administration of smaller doses did not increase gingival crevicular fluid Q10 levels to levels of detection. |
| Researcher | J. Soongswang |
| RESEARCHER AFFILIATION | Division of Cardiology, Department of Pediatrics, Faculty of Medicine, Siriraj Hospital, Mahidol University, Bangkok, Thailand. |
| PUBLICATION CITATION | Soongswang, J. et al. (2002). The effect of coenzyme Q10 on idiopathic dilated cardiomyopathy in children: A preliminary report. London, UK: Third Conference of the International Coenzyme Q10 Association. Poster. |
| TYPE OF STUDY | 9-month open-label prospective study |
| SAMPLE SIZE | 13 patients (10 female, 3 male) diagnosed with idiopathic chronic dilated cardiomyopathy with a median age of 4.4 years |
| DOSAGE | Bio-Quinone Q10 2.8 mg/kg/day for 9 months as a supplement to a conventional drug treatment |
| RESULTS | |
|
Used in addition to conventional anti-failure drugs, Q10 supplements improved NYHA functional class, admission rate, and ventricular depolarization rate in children with idiopathic dilated cardiomyopathy. |
| Researcher | M. Turunen |
| RESEARCHER AFFILIATION | Department of Biochemistry/Biophysics, Stockholm University, Stockholm, Sweden |
| PUBLICATION CITATION | Turunen, M., Wehlin, L., Sjöberg, M., Lundahl, J., Dallner, G., Brismar, K., & Sindelar, P. J. (2002). beta2-Integrin and lipid modifications indicate a non-antioxidant mechanism for the anti-atherogenic effect of dietary coenzyme Q10. Biochemical and Biophysical Research Communications, 296(2), 255-260. |
| TYPE OF STUDY | 10-week open-label study |
| SAMPLE SIZE | 10 healthy volunteers, 5 males and 5 females (52–68 years), none of them smokers, none of them on any other medications or dietary supplements |
| DOSAGE | Bio-Quinone Q10 200 mg for 10 weeks |
| RESULTS | |
|
Supplementation with 200 mg Q10 daily increased the levels of Q10 in plasma by 100 percent in the female volunteers and by 150 percent the male volunteers. Moreover, the alpha-tocopherol content in mononuclear and poly-nuclear cells increased continuously even though the volunteers were not taking a vitamin E supplement during the course of the study. The researchers interpreted the increase in alpha-tocopherol levels as evidence that Q10 increases vitamin E levels in human white blood cells and regenerates alpha-tocopherol radicals and protects them from destruction. Q10 supplementation in this study significantly decreased the extent of lipid modifications that are known to be a prerequisite for the development of atherosclerotic lesions. |
| Researcher | Raffaele Lodi |
| RESEARCHER AFFILIATION | Department of Biochemistry, University of Oxford, UK |
| PUBLICATION CITATION | Lodi, R., Hart, P. E., Rajagopalan, B., Taylor, D. J., Crilley, J. G., Bradley, J. L., & Cooper, J. M. (2001). Antioxidant treatment improves in vivo cardiac and skeletal muscle bioenergetics in patients with Friedreich's ataxia. Annals of Neurology, 49(5), 590-596. |
| TYPE OF STUDY | 6-month controlled study |
| SAMPLE SIZE | 10 Friedreich’s ataxia patients (5 males) with a mean age of 28 years compared with 10 healthy volunteers for the calf muscle and compared with 10 different healthy volunteers for the cardiac muscle |
| DOSAGE | Bio-Quinone Q10 400 mg/day and Vitamin E 2,100 international units/day |
| RESULTS | |
|
Treatment with Q10 and vitamin E for 6 months improved cellular bioenergetics significantly in Friedreich’s ataxia patients in cardiac muscle and in skeletal muscle. The treatment raised the International Cooperative Ataxia Rating Scale (ICARS) score in 6 of the 10 patients and narrowly failed to reach statistical significance following the 6 months of therapy. |
| Researcher | V. Hougaard Sunesen |
| RESEARCHER AFFILIATION | Department of Biochemistry and Nutrition, Technical University of Denmark, Lyngby, Denmark |
| PUBLICATION CITATION | Sunesen, V. H., Weber, C., & Hølmer, G. (2001). Lipophilic antioxidants and polyunsaturated fatty acids in lipoprotein classes: distribution and interaction. European Journal of Clinical Nutrition, 55(2), 115-123. |
| TYPE OF STUDY | Balanced three-period crossover study |
| SAMPLE SIZE | 18 apparently healthy, free-living, non-smoking volunteers (9 women and 9 men) with a mean age of 26 years plus/minus 3 years, all university students |
| DOSAGE | Three supplementation periods of 10 days: • Bio-Quinone 100 mg/day • Vitamin E (alpha-tocopherol) 350 mg/day • Concentrated fish oil 2 g/day |
| RESULTS | |
|
Fasting venous blood samples were collected twice before the first period and then after each period. Plasma and isolated lipoproteins were analyzed for concentrations of Coenzyme Q10, cholesterol, triacylglycerol, alpha and gamma-tocopherol, and fatty acids. Supplementation with Bio-Quinone Q10 significantly raised plasma Q10 levels from a mean level of 1.08 milligrams per liter to 2.44 milligrams per liter. Q10 increases occurred in all lipoprotein classes. The Q10 was primarily concentrated in the low-density lipoprotein. |
| Researcher | Meena Khatta |
| RESEARCHER AFFILIATION | University of Maryland School of Medicine, Baltimore, USA |
| PUBLICATION CITATION | Khatta, M., Alexander, B. S., Krichten, C. M., Fisher, M. L., Freudenberger, R., Robinson, S. W., & Gottlieb, S. S. (2000). The effect of coenzyme Q10 in patients with congestive heart failure. Annals of Internal Medicine, 132(8), 636-640. |
| TYPE OF STUDY | 6-month randomized, double-blind, controlled trial |
| SAMPLE SIZE | 55 patients who had congestive heart failure with New York Heart Association class III and IV symptoms (only 46 patients completed the study, 37 men and 9 women, average age: 64 years) |
| DOSAGE | Bio-Quinone Q10 200 mg/day |
| RESULTS | |
|
The results reported by the researchers: Coenzyme Q10 supplementation increased the mean serum Q10 levels from 0.95 +/- 0.62 mg/L to 2.2 +/- 1.2 mg/L. However, the Q10 supplementation did not significantly change the patients’ ejection fraction, peak oxygen consumption, and exercise duration. |
| Researcher | Jari Kaikkonen |
| RESEARCHER AFFILIATION | Research Institute of Public Health, University of Kuopio, Finland |
| PUBLICATION CITATION | Kaikkonen, J., Nyyssönen, K., Tomasi, A., Iannone, A., Tuomainen, T. P., Porkkala-Sarataho, E., & Salonen, J. T. (2000). Antioxidative efficacy of parallel and combined supplementation with coenzyme Q10 and d-alpha-tocopherol in mildly hypercholesterolemic subjects: a randomized placebo-controlled clinical study. Free Radical Research, 33(3), 329-340. |
| TYPE OF STUDY | Randomized, double-blind, placebo-controlled clinical trial |
| SAMPLE SIZE | 40 patients with mild hypercholesterolemia undergoing statin treatment |
| DOSAGE | Four groups tested for three months 1) Bio-Quinone Q10 200 mg daily … 2) Vitamin E (d-alpha-tocopherol) 700 mg daily 3) Both Bio-Quinone Q10 and Vitamin E daily … 4) Placebo daily |
| RESULTS | |
|
The researchers observed the following effects of supplementation: |
| Researcher | Fabrizio Mosca |
| RESEARCHER AFFILIATION | Institute of Biochemistry, University of Ancona Medical School, Ancona, Italy |
| PUBLICATION CITATION | Mosca, F., Balercia, G., Mantero, F., Arnaldi, G., & Littarru, G.P. (2000). Effect of Coenzyme Q10 supplementation on some biochemical parameters and on sperm motility in a group of asthenozoospermic patients. Frankfurt, Germany: Second Conference of the International Coenzyme Q10 Association, 157. |
| TYPE OF STUDY | An open-label uncontrolled study |
| SAMPLE SIZE | 7 asthenozoospermic patients (patients with reduced sperm motility) |
| DOSAGE | Bio-Quinone Q10 200 mg/day |
| RESULTS | |
|
The seminal plasma Q10 level rose significantly in all patients. The mean baseline value was 0.42 milligrams per liter. The post-supplementation value was 1.27 milligrams per liter. With Q10 supplementation, the ratio of sperm straight velocity to sperm curvilinear velocity increased. |
| Researcher | Branko Zorn |
| RESEARCHER AFFILIATION | Andrology Centre, Department of Obstetrics and Gynaecology, Slajmerjeva, Ljubljana, Slovenia |
| PUBLICATION CITATION | Zorn, B., Virant-Klun, I., Osredkar, J., & Krstic, N. (2000). The effects of a double-blind randomized placebo controlled cross-over trial using coenzyme Q10 (Bio-Quinone Q10) treatment on sperm quality and sperm in vitro fertilizing potential in infertile men. Frankfurt am Main, Germany: Second conference of the International Coenzyme Q10 Association. 126-127. |
| TYPE OF STUDY | Randomized, double-blind, placebo-controlled cross-over trial |
| SAMPLE SIZE | 60 infertile men |
| DOSAGE | Bio-Quinone Q10 (ubiquinol form) 100 mg once a day |
| RESULTS | |
|
Treatment with Q10 in the form of ubiquinol was not associated with significant changes in commonly measured sperm parameters. However, the treatment with Q10 – ubiquinol form was observed to decrease the percentage of sperm with abnormal DNA. The researchers related this effect to the antioxidant function of Coenzyme Q10. |
| Researcher | J. G. Eriksson |
| RESEARCHER AFFILIATION | Department of Epidemiology and Health Promotion, Helsinki, Finland. |
| PUBLICATION CITATION | Eriksson, J. G., Forsén, T. J., Mortensen, S. A., & Rohde, M. (1999). The effect of coenzyme Q10 administration on metabolic control in patients with type 2 diabetes mellitus. Biofactors (Oxford, England), 9(2-4), 315-318. |
| TYPE OF STUDY | 6-month randomized, double-blind, placebo-controlled clinical trial |
| SAMPLE SIZE | 23 type-2 diabetic patients |
| DOSAGE | Bio-Quinone 100 mg Q10 twice a day |
| RESULTS | |
|
Supplementation with 100 mg of Bio-Quinone Q10 twice daily caused serum Q10 levels to rise to levels more than three times the baseline levels. There was no observed correlation observed between serum Q10 levels and metabolic control. Q10 supplementation caused no significant changes in metabolic parameters. The patients tolerated the Q10 treatment well. It did not interfere with glycemic control. Consequently, it should be safe for type 2 diabetics to receive Q10 supplements as adjuvant treatment for heart disease. |
| Researcher | J. E. Henriksen |
| RESEARCHER AFFILIATION | The Diabetes Research Centre, Odense University Hospital, Denmark. |
| PUBLICATION CITATION | Henriksen, J. E., Andersen, C. B., Hother-Nielsen, O., Vaag, A., Mortensen, S. A., & Beck-Nielsen, H. (1999). Impact of ubiquinone (coenzyme Q10) treatment on glycemic control, insulin requirement and well-being in patients with Type 1 diabetes mellitus. Diabetic Medicine: A Journal of the British Diabetic Association, 16(4), 312-318. |
| TYPE OF STUDY | 3-month randomized, double-blind, placebo-controlled study |
| SAMPLE SIZE | 34 patients with Type 1 diabetes mellitus. |
| DOSAGE | Bio-Quinone 100 mg Q10 daily |
| RESULTS | |
|
At the beginning of the trial, there were no differences between the Q10 and the placebo groups with respect to age, body mass index, HbA1c levels (glycated hemoglobin levels = a measure of plasma glucose concentration), or daily insulin dose. During the course of the study, the serum Q10 levels increased in the Q10 supplementation group but not in the placebo group. The supplementation with Q10 did not cause any differences between the two groups in HbA1c levels, mean daily blood glucose levels, mean insulin dose needed, hypoglycemic episodes per week, cholesterol levels, or patient well-being The Q10 supplementation did not improve glycemic control, and it did not reduce insulin requirement. Conclusion: Q10 can be taken by patients with Type 1 diabetes without any obvious risk of hypoglycemia. |
| Researcher | Henrik Munkholm |
| RESEARCHER AFFILIATION | Department of Cardiology, Aalborg Hospital, Denmark |
| PUBLICATION CITATION | Munkholm, H., Hansen, H. H., & Rasmussen, K. (1999). Coenzyme Q10 treatment in serious heart failure. Biofactors (Oxford, England), 9(2-4), 285-289. |
| TYPE OF STUDY | 12-week randomized, double-blind, placebo-controlled investigation |
| SAMPLE SIZE | 22 patients with mean left ventricular ejection fraction 26%, with mean left ventricular internal diameter 71 mm, and with NYHA class II or III |
| DOSAGE | Bio-Quinone Q10 100 mg twice daily |
| RESULTS | |
|
Q10 supplementation was associated with significant improvement of the following outcomes: |
| Researcher | S. Hodges |
| RESEARCHER AFFILIATION | University of Sheffield, UK |
| PUBLICATION CITATION | Hodges, S., Hertz, N., Lockwood, K., & Lister, R. (1999). CoQ10: could it have a role in cancer management? Biofactors (Oxford, England), 9(2-4), 365-370. |
| TYPE OF STUDY | Open-label pilot study |
| SAMPLE SIZE | 6 cancer patients |
| DOSAGE | A combination of nutritional antioxidants: Bio-Quinone Q10 90 mg per day, 2850 mg Vitamin C, 2500 i.u. Vitamin E, 32.5 i.u. beta-carotene, 387 micrograms selenium, 1.2 g gamma-linolenic acid, and 3.5 g omega-3 fatty acids |
| RESULTS | |
|
In some of the patients, the Q10 and antioxidant therapy, used in addition to conventional therapy, was seen to reduce levels of TNF-alpha (Tumor Necrosis Factor, a cell-signaling protein produced by activated macrophages and involved in systemic inflammation). The researchers speculated that Q10 therapy might help in the inhibition of tumor-associated cytokines (proteins important in cell signaling). |
| Researcher | N. Denny |
| RESEARCHER AFFILIATION | School of Dentistry, University of Birmingham, Birmingham, UK |
| PUBLICATION CITATION | Denny, N., Chapple, I., & Matthews, J. (1999). Antioxidant and anti-inflammatory effects of coenzyme Q10: a preliminary study. Vancouver, British Columbia, Canada: International Association of Dental Research, Annual Meeting, Conference Paper Abstract, 193. |
| TYPE OF STUDY | 28-day randomized, double-blind, placebo-controlled, cross-over pilot study |
| SAMPLE SIZE | 10 non-smoking healthy volunteers, not using any vitamin supplements or medication, all diagnosed with mild to moderate gingival inflammation |
| DOSAGE | Bio-Quinone Q10 90 mg/day |
| RESULTS | |
|
Q10 treatment for 28 days reduced gingival bleeding indices significantly. Placebo treatment for 28 days did not. The data from the study indicate that Q10 supplementation may reduce gingival inflammation without necessarily raising Q10 concentrations in gingival crevicular fluid significantly. |
| Researcher | J. P. Watson |
| RESEARCHER AFFILIATION | Centre for Liver Research, University of Newcastle upon Tyne, UK |
| PUBLICATION CITATION | Watson, J. P., Jones, D. E., James, O. F., Cann, P. A., & Bramble, M. G. (1999). Case report: oral antioxidant therapy for the treatment of primary biliary cirrhosis: a pilot study. Journal of Gastroenterology and Hepatology, 14(10), 1034-1040. |
| TYPE OF STUDY | 3-month |
| SAMPLE SIZE | 24 patients diagnosed with primary biliary cirrhosis |
| DOSAGE | Group 1: compound antioxidant vitamin preparation Bio-Antox four tablets daily Group 2: Bio-Quinone Q10 100 mg together with Bio-Antox |
| RESULTS | |
|
Q10 supplementation coupled with antioxidant supplementation resulted in significant improvement in both pruritus (itching) and fatigue. The same improvement was not seen in the group supplemented with antioxidants alone. In the Q10 + antioxidants group, 9 of 13 patients reported less fatigue, and 10 of the 13 patients improved in at least one aspect of their Fisk Fatigue Severity Score. The combination of Q10 supplementation and antioxidant supplementation seems to relieve the symptoms of itching and fatigue in patients with primary biliary cirrhosis. |
| Researcher | D. Mari |
| RESEARCHER AFFILIATION | Clinical Oncology, University of Ancona Medical School, Ancona, Italy |
| PUBLICATION CITATION | Mari, D., Alleva, R., Tomasetti, M., Littarru, G.P. & Folkers, K. (1998). Reduction of chemotoxicity and enhancement of antioxidant capacity of plasma in cancer patients treated with conventional therapy plus CoQ10. Boston, Massachusetts: First Conference of the International Coenzyme Q10 Association. 132-133. |
| TYPE OF STUDY | 120-day randomized, open-label pilot study |
| SAMPLE SIZE | 58 cancer patients (42 male patients and 16 female patients) diagnosed with lung cancer (24), colon cancer (25), or breast cancer (9) |
| DOSAGE | Group A: Standard chemotherapy for the specific cancer (30 patients) Group B: Bio-Quinone Q10 300 mg/day in addition to the standard chemotherapy (28 patients) |
| RESULTS | |
|
Supplementation with Q10 increased plasma Q10 levels from 0.8 +/- 0.2 milligrams per liter to 5.5 +/- 2.5 milligrams per liter. Q10 supplementation restored low baseline total antioxidant capacity values in the cancer patients to normal levels. Moreover, there were 3 early deaths and 3 cases of IV grade toxicity in the group receiving the chemotherapy without Q10 supplementation. There were no early deaths and no cases of IV grade toxicity in the Q10 supplementation group. |
| Researcher | Louise Tholle |
| RESEARCHER AFFILIATION | Research Institute for Human Nutrition, Royal College of Veterinary and Agricultural Science, Hillerød, Denmark |
| PUBLICATION CITATION | Tholle L., Toubro S., Astrup A., & Toubro S. (1998). Effect of Q10 on resting energy expenditure in males. International Journal of Obesity, 23,3 (Suppl), S158. |
| TYPE OF STUDY | Randomized, double-blind, placebo-controlled, cross-over study |
| SAMPLE SIZE | 11 healthy male subjects with ages ranging from 52 to 70 years |
| DOSAGE | Bio-Quinone Q10 100 mg daily |
| RESULTS | |
|
Supplementation with 100 mg of Bio-Quinone Q10 for one week increased plasma Q10 concentration by 79 % from 1.17 micromol/liter to 2.10 micromol/liter. After one week of Q10 treatment, 8 of 11 subjects were able to name the correct treatment (P< 0.08) because of a better feeling of well-being and because of a reduction in the need for sleep. |
| Researcher | R. Aejmelaeus |
| RESEARCHER AFFILIATION | Depattment of Pharmacology, Clinical Pharmacology and Toxicology, Medical School, University of Tampere, Tampere, Finland |
| PUBLICATION CITATION | Aejmelaeus, R., Metsä-Ketelä, T., Laippala, P., Alho, H. & Solakvi, T. (1997). Ubiquinol-10 and total peroxyl radical trapping capacity of LDL lipoproteins during aging: the effects of Q-10 supplementation. Molecular Aspects of Medicine, 18, s113-s120. |
| TYPE OF STUDY | Comparative study |
| SAMPLE SIZE | 70 healthy volunteers, aged 28-77 years, 32 male and 38 female, non-smokers and non-users of medicines and dietary supplements |
| DOSAGE | Bio-Quinone Q10 100 mg (administered to 17 volunteers) |
| RESULTS | |
|
The researchers used a luminescent method to measure the peroxyl radical trapping capacity of human LDL. Previous research had shown that, in the Finnish male population, the peroxyl radical trapping capacity declines with age. This study’s results show that LDL antioxidant defenses decrease with age in the Finnish male population and that the decline is most significant in males under 50 years. In older age groups, the LDL antioxidant defense values remain stable at a low level. Supplementation with Q10 doubled the number of ubiquinol-containing LDL molecules and could play a role in inhibiting LDL oxidation. A highly significant increase in LDL-ubiquinol levels was detected in the 17 volunteers who took the Coenzyme Q10 100 mg supplement daily. |
| Researcher | Aby Lewin |
| RESEARCHER AFFILIATION | Department of Obstetrics and Gynecology, Hadassah-Hebrew University Medical School, Jerusalem, Israel |
| PUBLICATION CITATION | Lewin, A. & Lavon, H. (1997). The Effect of Coenzyme Q10 on sperm motility and function. Molecular Aspects of Medicine, 18 (Supplement), s213-s219. |
| TYPE OF STUDY | Open-label pilot study |
| SAMPLE SIZE | 17 patients with low fertilization rates after ICSI (intracytoplasmic sperm injection) |
| DOSAGE | Bio-Quinone Q10 60 mg/day, for a mean of 103 days |
| RESULTS | |
|
The researchers did not observe any significant change in most sperm parameters, but they did see a significant improvement in fertilization rates after the Q10 treatment. The researchers concluded that treatment with Q10 can result in improvement in sperm functions in selective patients. |
| Researcher | T. Ylikoski |
| RESEARCHER AFFILIATION | Vuokatti Sports Testcenter, Finland |
| PUBLICATION CITATION | Ylikoski, T., Piirainen, J., Hanninen, O., & Penttinen, J. (1997). The effect of coenzyme Q10 on the exercise performance of cross-country skiers. Molecular Aspects of Medicine, 18 SupplS283-S290. |
| TYPE OF STUDY | Double-blind cross-over study |
| SAMPLE SIZE | 25 Finnish top-level cross-country skiers |
| DOSAGE | Bio-Quinone 90 mg of Q10 per day |
| RESULTS | |
|
Q10 supplementation led to significantly improved aerobic and anaerobic threshold and maximal oxygen consumption in the elite cross-country skiers. Moreover, 94% of the Finnish skiers enrolled in the study indicated the Q10 supplement had helped to improve their performance and their recovery time. Only 33% of the participants felt the same during the placebo periods. |
| Researcher | V.L. Serebruany |
| RESEARCHER AFFILIATION | Heart Associates Research and Education Foundation, Baltimore, MD, USA |
| PUBLICATION CITATION | Serebruany, V. L., Gurbel, P. A., Ordoñez, J. V., Herzog, W. R., Rohde, M., Mortensen, S. A., & Folkers, K. (1997). Could coenzyme Q10 affect hemostasis by inhibiting platelet vitronectin (CD51/CD61) receptor? Molecular Aspects of Medicine, 18 SupplS189-S194. |
| TYPE OF STUDY | 20-day open-label study |
| SAMPLE SIZE | 15 indivduals |
| DOSAGE | Bio-Quinone Q10 100 mg twice daily |
| RESULTS | |
|
The researchers had hypothesized that supplementation with Q10 might affect hemostasis (the process of stopping bleeding, the first step of wound healing) by inhibiting the formation of platelets and might, therefore, play a role in the diminishing of the incidence of thrombosis in platelets. With the Q10 treatment, the researchers found a 20.2% decrease in plasma fibronectin (a protein component of blood plasma), a 20.6% decrease in thromboxane B2 (a lipid metabolite of the blood clotting process), a 23.2% decrease in prostacyclin (an inhibitor of platelet activation), and a 17.9% decrease in endothelin-1 (a peptide that constricts blood vessels and raises blood pressure). This study provides direct evidence of a link between Q10 supplementation, platelet activation, and hemostasis. |
| Researcher | Jari Kaikkonen |
| RESEARCHER AFFILIATION | Research Institute of Public Health, University of Kuopio, Finland |
| PUBLICATION CITATION | Kaikkonen, J., Nyyssönen, K., Porkkala-Sarataho, E., Poulsen, H. E., Metsä-Ketelä, T., Hayn, M., & ... Salonen, J. T. (1997). Effect of oral coenzyme Q10 supplementation on the oxidation resistance of human VLDL+LDL fraction: absorption and antioxidative properties of oil and granule-based preparations. Free Radical Biology & Medicine, 22(7), 1195-1202. |
| TYPE OF STUDY | 2-month randomized, double-blind, placebo-controlled clinical trial |
| SAMPLE SIZE | 60 healthy men, smokers, mean age 46 +/- 7 years |
| DOSAGE | Bio-Quinone 90 mg/day |
| RESULTS | |
|
The oil-based Bio-Quinone Q10 increased the plasma Q10 levels by 178% and increased the level of Q10 in the VLDL+LDL fraction by 160%. However, the Q10 supplementation was not seen to increase the oxidation resistance of the VLDL+LDL fraction, when the extent of copper-induced VLDL+LDL oxidation and haemin+H(2)O(2)-induced VLDL+LDL oxidation was assessed. There was no significant improvement in the levels of plasma malondialdehyde (a bio-marker for oxidative damage). |
| Researcher | M. Mizuno |
| RESEARCHER AFFILIATION | Department of Medical Biochemistry and Genetics, Panum Institute, University of Copenhagen, Denmark |
| PUBLICATION CITATION | Mizuno, M., Quistorff, B., Theorell, H., Theorell, M., & Chance, B. (1997). Effects of oral supplementation of coenzyme Q10 on 31P-NMR detected skeletal muscle energy metabolism in middle-aged post-polio subjects and normal volunteers. Molecular Aspects of Medicine, 18 SupplS291-S298. |
| TYPE OF STUDY | 6-month open-label controlled pilot study |
| SAMPLE SIZE | 7 middle-aged subjects, 3 post-polio patients and 4 healthy individuals |
| DOSAGE | Bio-Quinone Q10 100 mg/day |
| RESULTS | |
|
Supplementation with Q10 resulted in a progressive decrease in the resting Pi/PCr ratio (ratio of the phosphorous metabolites, inorganic phosphorous to phosphocreatine) in the post-polio patients and in less pronounced changes in the control subjects. Post-polio patients also registered a decrease in muscle pH, following Q10 supplementation. No such decrease was seen in the healthy subjects. Q10 supplementation did result in a decreased half-time of recovery for PCr (phosphocreatine) in both groups. Based on the results of this study, Q10 supplementation seems to affect muscle energy metabolism in post-polio subjects more than in healthy control subjects. |
| Researcher | E. Strijks |
| RESEARCHER AFFILIATION | Department of Neurology, University Hospital Nijmegen, The Netherlands |
| PUBLICATION CITATION | Strijks, E., Kremer, H. P., & Horstink, M. W. (1997). Q10 therapy in patients with idiopathic Parkinson's disease. Molecular Aspects of Medicine, 18 SupplS237-S240. |
| TYPE OF STUDY | 3-month open-label trial |
| SAMPLE SIZE | 10 patients with Parkinson disease |
| DOSAGE | Bio-Quinone Q10 200 mg per day |
| RESULTS | |
|
There was no significant improvement in motor performance. |
| Researcher | Christine Weber |
| RESEARCHER AFFILIATION | Department of Biochemistry and Nutrition, Technical University of Denmark, Lyngby, Denmark |
| PUBLICATION CITATION | Weber, C., & Bysted, A. H. (1997). Intestinal absorption of Coenzyme Q10 administered in a meal or as capsules to healthy subjects. Nutrition Research, 17, 6, 941-945. |
| TYPE OF STUDY | Randomized cross-over study |
| SAMPLE SIZE | 9 healthy non-smoking male volunteers with an average age of 22 years |
| DOSAGE | Bio-Quinone Q10 30 mg |
| RESULTS | |
|
To investigate the bioavailability of dietary Coenzyme Q10 in humans, the researchers administered single doses of Q10 (30 mg/person) either as a meal of cooked pork heart or as 30 mg Q10 capsules. The increase in serum Q10 levels reached a maximum peak at six hours after the ingestion of either the meal or the capsules. The pork heart meal raised serum Q10 levels significantly from 0.97 mg/L to 1.44 mg/L. The supplementation with Q10 capsules raised the serum Ql0 concentrations significantly from a baseline level of 0.88 mg/L to 1.19 mg/L. There was no statistically significant difference between the Q10 absorption from the meal and from the capsules. |
| Researcher | Chris Alford |
| RESEARCHER AFFILIATION | University of the West of England, Bristol, UK |
| PUBLICATION CITATION | Alford, C., Service, J., & Hogan, J. (1996). The effects of food supplements (ubiquinone and selenium) on mood and compliance. Cambridge, UK: British Association for Psychopharmacology. Abstract. |
| TYPE OF STUDY | 5-week randomized, double-blind, placebo-controlled parallel study |
| SAMPLE SIZE | Four groups of 10 subjects each (age range: 18-23 years) |
| DOSAGE | Bio-Quinone Q10 60 mg/day and Bio-Selenium 100 micrograms/day |
| RESULTS | |
|
Supplementation with ubiquinone and supplementation with selenium were both associated with better compliance with the study regimen and with improved energy levels and improved mood states. The results were measured using the Visual Analogue Scales scores (for energy) and UWIST Mood Adjective Checklist scores. |
| Researcher | H. Alho |
| RESEARCHER AFFILIATION | School of Medicine, University of Tampere, Tampere, Finland |
| PUBLICATION CITATION | Alho, H., Lönnrot, K., & Aejmelaeus, R. (1996). Ubiquinone and total peroxyl radical trapping capacity of LDL lipoproteins during aging: the effects of Q10 supplementation. 9th International Symposium on Biomedical and Clinical Aspects of Coenzyme Q10, 9, 20. |
| TYPE OF STUDY | Open-label study |
| SAMPLE SIZE | 86 healthy subjects aged 25 to 81 years |
| DOSAGE | Bio-Quinone Q10 100 mg per day |
| RESULTS | |
|
The researchers studied the total peroxyl-radical trapping capacity (TRAP-LDL) in plasma LDL. In female subjects, the TRAP-LDL trapping capacity tended to decrease with age. In males, above the age of 25, a decrease in TRAP-LDL trapping capacity was significantly associated with increasing age. The decreases in TRAP-LDL trapping capacity were associated with decreasing levels of ubiquinol, the reduced form of Coenzyme Q10, in plasma. Supplementation with Q10 raised, significantly, the levels of ubiquinol in plasma, and ubiquinol is the form of Coenzyme Q10 that is a potent antioxidant. |
| Researcher | K. Lönnrot |
| RESEARCHER AFFILIATION | Department of Biomedical Sciences, University of Tampere, Finland |
| PUBLICATION CITATION | Lönnrot, K., Metsä-Ketelä, T., Molnár, G., Ahonen, J. P., Latvala, M., Peltola, J., & ... Alho, H. (1996). The effect of ascorbate and ubiquinone supplementation on plasma and CSF total antioxidant capacity. Free Radical Biology & Medicine, 21(2), 211-217. |
| TYPE OF STUDY | 4-week open-label study |
| SAMPLE SIZE | 5 healthy volunteers |
| DOSAGE | Bio-Quinone Q-10 100 mg/day the first 2 weeks and 300 mg/day the next 2 weeks Ascorbic acid 500 mg/day the first 2 weeks and 1,000 mg/day the next 2 weeks |
| RESULTS | |
|
With supplementation, the plasma Q10 levels rose from a mean baseline value of 0.57 mg/L to a mean value of 2.13 mg/L over the course of the four-week study. |
| Researcher | M. Mizuno |
| RESEARCHER AFFILIATION | Department of Medical Biochemistry and Genetics, Panum Institute, University of Copenhagen, Denmark |
| PUBLICATION CITATION | Mizuno, M., Nielsen, A. N., Ratkevicius, A., Mohr, T., Rohde, M., Mortensen, S. A., & Quistorff B. (1996). CoQ10 supplementation does not affect maximal oxygen uptake, 31 P-NMR detected metabolism, or muscle fatigue in endurance athletes. 9th International. Symposium on. Biomedical and Clinical Aspects of Coenzyme Q10, 9, 80-81. |
| TYPE OF STUDY | 6-week open-label study |
| SAMPLE SIZE | 7 healthy endurance athletes |
| DOSAGE | Bio-Quinone Q10 100 mg per day |
| RESULTS | |
|
Supplementation with 100 mg of Q10 per day raised serum Q10 levels from a mean of 0.92 micrograms per milliliter to 1.93 micrograms per milliliter. |
| Researcher | Magnus Nylander |
| RESEARCHER AFFILIATION | Clinical Research Centre at Novum, Huddinge Hospital, Karolinska Institutet, Stockholm, Sweden |
| PUBLICATION CITATION | Nylander, M., Weiner, J., & Nordlund, M. (1996). A double-blind clinical dose-response study on effects of CoQ10 on gingival bleeding/periodontal disease in ordinary people. Helsinki, Finland: 7th International Symposium on Trends in Biomedicine in Finland, Suppl 8, 1-7. |
| TYPE OF STUDY | Randomized, double-blind, placebo-controlled, dose-response study |
| SAMPLE SIZE | 60 ordinary non-smoking Swedish adults randomized to three groups: • 17 subjects receiving a daily dose of 30 mg for 10 days • 19 subjects receiving a daily dose of 100 mg • 24 subjects receiving placebo capsules |
| DOSAGE | Bio-Quinone Q10 30 mg/day or 100 mg/day |
| RESULTS | |
|
The researchers recorded gingival bleeding at the start and at the end of the study. They observed the greatest decrease in gingival bleeding in the group that was received 100 mg of Q10 daily and the least decrease in gingival bleeding in the placebo group. They found a statistically significant difference between the group that received 100 mg of Q10 daily and the other two groups (30 mg Ql0 and placebo) in the extent of improvement in gingival bleeding points. |
| Researcher | J. T. Salonen |
| RESEARCHER AFFILIATION | Research Institute of Public Health, University of Kuopio, Finland |
| PUBLICATION CITATION | Salonen, J. T., Kaikkonen, J., Nyyssönen, K.,, Maijala, L., Porkkala-Sarataho, E., Salonen, R., & Korpela, H. (1996). Coenzyme Q10 supplementation and lipoprotein oxidation resistance: a randomized placebo controlled double blind study in marathon runners. Ancona, Italy: 9th International Symposium on Biomedical and Clinical Aspects of Coenzyme Q10. 23. |
| TYPE OF STUDY | 3-week randomized, double-blind, placebo-controlled study |
| SAMPLE SIZE | 37 moderately trained healthy marathon runners |
| DOSAGE | Bio-Quinone Q10 90 mg/day |
| RESULTS | |
|
Supplementation with Q10 significantly increased plasma Q10 levels as compared to placebo. Q10 supplementation also significantly increased measured oxidation resistance (measuring copper-induced VLDL and LDL oxidation lag time). There was no significant difference in the extent of muscle damage (measured by serum creatine kinase activity) or muscle metabolites (measured by plasma lactates). |
| Researcher | D. P. Taggart |
| RESEARCHER AFFILIATION | Departments of Cardiothoracic Surgery and Biochemistry, Royal Brompton Hospital, London, England |
| PUBLICATION CITATION | Taggart, D. P., Jenkins, M., Hooper, J., Hadjinikolas, L., Kemp, M., Hue, D., & Bennett, G. (1996). Effects of short-term supplementation with coenzyme Q10 on myocardial protection during cardiac operations. The Annals of Thoracic Surgery, 61(3), 829-833. |
| TYPE OF STUDY | Short-term randomized, double-blind, placebo-controlled prospective study |
| SAMPLE SIZE | 20 patients with well-preserved left ventricular function scheduled to undergo elective coronary revascularization |
| DOSAGE | Bio-Quinone Q10 600 mg in divided doses 12 hours before operation |
| RESULTS | |
|
Following the patients’ heart surgeries, the researchers monitored the levels of myoglobin (a protein released more rapidly by an infarcted heart), creatine kinase MD fraction (a bio-marker for heart muscle injury), and cardiac troponin T (an indicator of heart muscle injury) for 1, 6, 24, 72, and 120 hours following the operations. Patients in both groups registered significant post-operative increases in myoglobin, kinase MB fraction, and cardiac troponin T levels. Short-term Q10 supplementation did not make a significant difference in the levels of these bio-markers. |
| Researcher | Renata Alleva |
| RESEARCHER AFFILIATION | Institute of Biochemistry, Faculty of Medicine, University of Ancona, Ancona, Italy |
| PUBLICATION CITATION | Alleva, R., Tomasetti, M., Battino, M., Curatola, G., Littarru, G. P., & Folkers, K. (1995). The roles of coenzyme Q10 and vitamin E on the peroxidation of human low density lipoprotein sub-fractions. Proceedings of the National Academy of Sciences of the United States of America, 92(20), 9388-9391. |
| TYPE OF STUDY | Open-label study of the relationship between the Coenzyme Q10 levels and levels of free radical hydro-peroxide in three sub-fractions of low-density lipoproteins |
| SAMPLE SIZE | 10 healthy male adult volunteers with a mean age of 26 years |
| DOSAGE | Bio-Quinone Q10 100 mg per day for 30 days |
| RESULTS | |
|
The researchers knew that elevated levels of lipoprotein sub-fraction LDL-3 are frequently seen in patients with coronary artery disease or at risk for coronary artery disease. They hypothesized that the higher susceptibility to oxidation of LDL-3 might be responsible for the increased risk of coronary artery disease. |
| Researcher | R. Laaksonen |
| RESEARCHER AFFILIATION | Department of Clinical Pharmacology, University of Helsinki, Helsinki, Finland |
| PUBLICATION CITATION | Laaksonen, R., Fogelholm, M., Himberg, J. J., Laakso, J., & Salorinne, Y. (1995). Ubiquinone supplementation and exercise capacity in trained young and older men. European Journal of Applied Physiology and Occupational Physiology, 72(1-2), 95-100. |
| TYPE OF STUDY | 6-week double-blind, placebo-controlled, cross-over study |
| SAMPLE SIZE | 19 physically fit men, 11 young men (aged 22-38 years) and 8 older men (aged 60-74 years) |
| DOSAGE | Bio-Quinone Q10 120 mg/day |
| RESULTS | |
|
Q10 supplementation increased serum ubiquinone levels significantly in both age groups. The serum Q10 concentrations were higher in the older subjects during the entire study, and the older athletes responded better than the younger athletes to the Q10 treatment. The Q10 supplementation did not improve the athletes’ aerobic endurance capacity. It may be that a greater dosage is needed in healthy, physically fit people. A different study (Kamikawa et al., 1985) has shown that Q10 supplementation does improve maximal exercise capacity in patients with angina pectoris. |
| Researcher | Karl Folkers |
| RESEARCHER AFFILIATION | Institute for Biomedical Research, University of Texas at Austin, USA |
| PUBLICATION CITATION | Folkers, K., Moesgaard, S., & Morita, M. (1994). A one-year bioavailability study of coenzyme Q10 with 3 months withdrawal period. Molecular Aspects of Medicine, 15 Suppls281-s285. |
| TYPE OF STUDY | One-year bioavailability study with nine months of supplementation and three months of withdrawal |
| SAMPLE SIZE | 21 healthy individuals |
| DOSAGE | Bio-Quinone Q10 30 mg three times a day |
| RESULTS | |
|
The researchers took blood samples and tested Q10 levels at baseline, after three and nine months of supplementation, and three months after withdrawal. The average Q10 levels rose from approximately one milligram per liter prior to supplementation to approximately two milligrams per liter after three and nine months of supplementation. The Q10 levels returned to the pretreatment level after withdrawal. |
| Researcher | Knud Lockwood |
| RESEARCHER AFFILIATION | Private Outpatient Clinic, Copenhagen, Denmark |
| PUBLICATION CITATION | Lockwood, K., Moesgaard, S., & Folkers, K. (1994). Partial and complete regression of breast cancer in patients in relation to dosage of coenzyme Q10. Biochemical and Biophysical Research Communications, 199(3), 1504-1508. |
| TYPE OF STUDY | 18-month Adjuvant Nutritional Intervention in Cancer protocol |
| SAMPLE SIZE | 32 patients with breast cancer, aged 32-81 years and classified high risk because of tumor spread to the lymph nodes in the armpit |
| DOSAGE | A combination of nutritional antioxidants: Bio-Quinone Q10 90 mg per day, 2850 mg Vitamin C, 2500 i.u. Vitamin E, 32.5 i.u. beta-carotene, 387 micrograms selenium, 1.2 g gamma-linolenic acid, and 3.5 g omega-3 fatty acids |
| RESULTS | |
|
The researchers made the following observations during the study period: • No patients died even though the expected number to die was four. |
| Researcher | Bodo Kuklinski |
| RESEARCHER AFFILIATION | Klinik für Innere Medizin (Department of Internal Medicine), Rostock, Germany |
| PUBLICATION CITATION | Kuklinski, B., Weissenbacher, E., & Fähnrich, A. (1994). Coenzyme Q10 and antioxidants in acute myocardial infarction. Molecular Aspects of Medicine, 15 Suppls143-s147. |
| TYPE OF STUDY | Randomized, double-blind, placebo-controlled study |
| SAMPLE SIZE | 61 patients admitted with acute myocardial infarction and with symptoms of less than six hours’ duration (32 patients in the treatment group, 29 patients in the control group) |
| DOSAGE | Bio-Quinone Q10 100 mg and Bio-Selenium 100 micrograms for a period of one year |
| RESULTS | |
|
None of the patients in the Q10/selenium group showed a prolongation of the frequency corrected QT-interval (the time between the start of the Q wave and the end of the T wave in the heart's electrical cycle) whereas 40% of the patients in the placebo group showed a prolongation of the QT-interval by more than 440 msec (p < 0.001). The researchers observed no significant differences with respect to early complications. However, during the one-year follow-up period after the myocardial infarction, six patients (20%) in the placebo group died from re-infarction, but only one patient (3%) in the Q10/selenium group died, and that death was from a non-cardiac cause. |
| Researcher | Malene Weis |
| RESEARCHER AFFILIATION | Royal Danish School of Pharmacy, Department of Pharmaceutics, Denmark |
| PUBLICATION CITATION | Weis, M., Mortensen, S. A., Rassing, M. R., Møller-Sonnergaard, J., Poulsen, G., & Rasmussen, S. N. (1994). Bioavailability of four oral coenzyme Q10 formulations in healthy volunteers. Molecular Aspects of Medicine, 15 Suppls273-s280. |
| TYPE OF STUDY | Four-way randomized cross-over trial |
| SAMPLE SIZE | 10 healthy volunteers |
| DOSAGE | Various formulations of Coenzyme Q10 100 mg |
| RESULTS | |
|
The study of four different formulations of Q10 revealed the following information: |
| Researcher | Christine Weber |
| RESEARCHER AFFILIATION | Department of Biochemistry and Nutrition, Technical University of Denmark, Lyngby, Denmark |
| PUBLICATION CITATION | Weber, C., Sejersgård Jakobsen, T., Mortensen, S. A., Paulsen, G., & Hølmer, G. (1994). Antioxidative effect of dietary coenzyme Q10 in human blood plasma. International Journal for Vitamin and Nutrition Research 64(4), 311-315. |
| TYPE OF STUDY | Open-label study |
| SAMPLE SIZE | 22 healthy young subjects (9 men and 13 women) |
| DOSAGE | Bio-Quinone Q10 90 mg/day |
| RESULTS | |
|
One week of supplementation with 90 mg/day of Bio-Quinone Q10 increased plasma Q10 levels significantly from 0.7 +/- 0.1 micromol/l before supplementation to 1.7 +/- 0.3 micromol/l. The level of thio-barbituric acid reactive substances (TBARS), which formed as a byproduct of lipid peroxidation, decreased during the first two weeks of Q10 supplementation. The researchers concluded that the decrease of TBARS and the presence of most of the orally supplemented Q10 in the reduced form in plasma seemed to indicate an anti-oxidative role for Q10 in the blood. |
| Researcher | Svend Aage Mortensen |
| RESEARCHER AFFILIATION | Department of Cardiology and Internal Medicine, Copenhagen University Hospital, Copenhagen, Denmark |
| PUBLICATION CITATION | Mortensen, S. A. (1993). Perspectives on therapy of cardiovascular diseases with coenzyme Q10 (ubiquinone). The Clinical Investigator, 71(8 Suppl), S116-S123. |
| TYPE OF STUDY | Open-label study |
| SAMPLE SIZE | 5 patients diagnosed with cardiomyopathy |
| DOSAGE | Bio-Quinone Q10 100 mg per for a mean of 5 months |
| RESULTS | |
|
Supplementation with 100 mg daily of the Bio-Quinone Q10 raised Coenzyme Q10 levels in blood from a mean level of 0.66 milligrams per liter to a mean of 1.50 milligrams per liter. Increasing blood and tissue levels of Q10 is important because it is known that blood and tissue levels of Q10 are significantly lower in patients with advanced heart failure (NYHA classes III and IV) as compared with patients with milder stages of heart failure (NYHA classes I and II) (Mortensen, 1993, p. S117). |
| Researcher | Magnus Nylander |
| RESEARCHER AFFILIATION | The Institution for Hygiene, Karolinska Institute, Stockholm, Sweden |
| PUBLICATION CITATION | Nylander, M. (1991). Kliniska effekter på kostsupplementering med ubiquinon, coensym Q10 (Clinical effects of dietary supplementation with ubiquinone Coezyme Q10). BIOMED, 4, 6-11. |
| TYPE OF STUDY | Open-label preliminary study |
| SAMPLE SIZE | 6 people with varying degree of periodontitis |
| DOSAGE | Bio-Quinone Q10 30 mg/day and 100 mg/day for 6 – 12 weeks |
| RESULTS | |
|
The results of this preliminary study indicate that supplementation with Q10 can reduce the extent of bleeding tendency and inflammation of the gingiva in individuals diagnosed with periodontitis. The dosage and the amount of time needed for the supplementation to give a therapeutic effect varied from one individual to another. |
| Researcher | Stefano Raffaele Giannubilo |
| RESEARCHER AFFILIATION | Department of Clinical Sciences, Polytechnic University of Marche, 60123 Ancona, Italy |
| PUBLICATION CITATION | Giannubilo SR, Orlando P, Silvestri S, Cirilli I, Marcheggiani F, Ciavattini A, Tiano L. (2018). CoQ10 Supplementation in Patients Undergoing IVF-ET: The Relationship with Follicular Fluid Content and Oocyte Maturity. Antioxidants (Basel);7(10):141. |
| TYPE OF STUDY | Open-label trial |
| SAMPLE SIZE | 30 patients undergoing IVF–ET program |
| DOSAGE | Myoqinon 200 mg/day |
| RESULTS | |
|
The researcher’s observation leads to the hypothesis that the oral supplementation of CoQ10 may improve follicular fluid oxidative metabolism and oocyte quality, specially in over 35-year-old women |

